Fight Hidden Corrosion in Stainless-Steel Molds
Stainless-steel tooling is used in 10% to 20% of all molding jobs and is suited expressly to harsh molding applications.
Stainless-steel tooling is used in 10% to 20% of all molding jobs and is suited expressly to harsh molding applications. Toolmakers select stainless steel for cores and cavities to combat the attack of corrosion from aggressive polymers like PVC and any by-products of resin degradation created during the molding process. Yet, stainless-steel molds are susceptible to a common type of corrosive attack and tool failure called stress corrosion cracking. It is irreversible and can ruin a brand- new mold in as little as three weeks or less. This phenomenon is not widely understood in the plastics industry, mainly because it starts at the microscopic level and reveals itself as cracks that are typically misinterpreted as a mechanical fracture instead of an event that is corrosion related.
Stress corrosion cracking is the formation of cracks caused by the combination of a corrosive environment inside the mold and the presence of tensile (stretching/flexing) forces. The cracks initiate at localized stressed sites and then propagate through the material, typically following the steel’s grain boundaries. When the cracks progress far enough into the steel, the stresses of molding are enough to continue the propagation or “movement” of the crack to the outer areas of the mold. Stress corrosion can occur even in mildly corrosive environments.
Recipe for mold failure
How can a mold made of stainless steel crack from corrosion when corrosion resistance is the main purpose for using the stainless mold steel? This type of corrosive attack is not due directly to the molding operation or anything occurring at the surface of the mold. The attack originates inside the tool —specifically in the water lines. The primary causal elements include the metallurgy of stainless steel, the presence of chlorine in the water used in the cooling lines of the mold, and the stresses on the tool during molding.
What gives stainless steel its corrosion resistance is the element chromium, which occurs in higher concentrations (13.5% to 16.5%) in stainless steel than in conventional tool steels. When the chromium oxidizes—a naturally occurring phenomenon—it forms chromium oxide. Unlike iron, which turns into flaky rust and falls off when oxidized, chromium oxide builds up into a layer as it degrades. When that layer reaches a certain thickness, chromium oxidation under the layer stops. That oxide layer provides the corrosion resistance of the stainless steel.
Mold stress helps create the environment for stress corrosion cracking. All molds flex or deflect in operation (during clamp up), and the deflection of the mold generates tensile stresses in the tool. There are other stresses in the mold, particularly in the areas around and within the water-cooling lines. Intersections of the water lines, threaded areas, and sharp corners or notches are areas where the tensile stress can be elevated (called a stress riser). In some instances a threaded plug used to seal a water- opening can generate enough stress to initiate stress corrosion cracking, even if the mold is not in use.
The third ingredient is chlorine, which exists in trace amounts in the mold cooling water. It is found in nearly all public water supplies. The presence of chlorine ions in the water combined with the deflection of the mold brings about a chemical reaction between the chlorine and chromium in the steel. The resulting product is chromium chloride, which is water soluble and thus dissolves out of the steel into the cooling water. First, the chlorine attacks the protective layer of chromium oxide, then it depletes nonoxidized chromium within the steel. This chemical reaction, together with the stresses of molding, then produces fine cracks in the steel’s matrix.
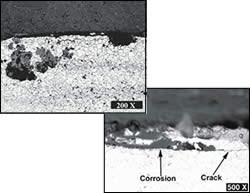
The chemical reaction between the chromium and chlorine tends to occur in areas where there are stress risers and where the chromium in the steel is at its highest. The highest concentrations of chromium carbides in stainless steel occur at the steel grain boundaries, which are formed during the heat treatment of the steel. That’s why the stress corrosion cracks are inter-granular and have a brittle appearance even if the material is ductile. It is this appearance that often causes stress corrosion cracking to be misinterpreted as a mechanical fracture.
The cracks themselves also become stress risers, and as the molding operation continues, the cracks propagate farther into the mold. The rate of crack propagation is influenced by the temperature of the cooling water, its chlorine content, and the stress in the mold. In molding, nearly any amount of deflection is enough to impart stress, even as little as 5 psi.
Control the cracking
The most devastating aspect of stress corrosion cracking is that by the time you realize it is happening the mold is cracked all the way through and is ruined. While the mold is losing its integrity, you can’t see it. If the problem was just on the surface, you would address it with polishing or plating.
Fortunately, there are actions a molder can take to protect the mold. Mold temperature can play a role in creating the corrosive environment. The higher the mold temperature the more likely a corrosive environment will be established. Keeping the water in the tool as cool as possible may reduce the chance of stress corrosion cracking.
Also, the diameter of the water cooling lines may have an impact, since large-diameter channels mean a larger channel surface area for corrosive attack to occur and a larger volume of water that can run through.
The pH level in the water is also critical. Low pH levels (high acidity) indicate a corrosive environment, which may be caused by evaporation of the water during molding, causing a build-up in concentration of acidity. Users may consider the purchase of a pH meter to test the acid levels in the water in order to keep them as low as possible. Molders can also talk with their local water utility representative about the issue of pH and chlorine. A water de-ionizer may help to minimize chlorine levels in the water.
What’s more, heat treating during production of the mold plays a role in corrosion protection. There are several ways to through-harden steels, and poor-quality heat treating can make the tool more susceptible. Preventing overheating during the hardening process can help to lessen the number of grain boundaries.
It’s also worth considering how to keep clamp pressures as low as possible during molding. The lower the stress levels, the less likely it is that stress corrosion damage will occur.
Ed Severson is the technical manager for plastics applications at Bohler-Uddeholm North America, a specialty steel manufacturer in Rolling Meadows, Ill. He can be reached at (847) 577-2220, ext. 477, or by e-mail: ed.severson@bucorp.com
Related Content
Hot Runners: A View from the Bottom Up
Addressing hot-runner benefits, improvements, and everyday issues from the perspective of decades of experience with probably every brand on the market. Part 1 of 2.
Read MoreWhy Shoulder Bolts Are Too Important to Ignore (Part 2)
Follow these tips and tricks for a better design.
Read MoreHow to Optimize Pack & Hold Times for Hot-Runner & Valve-Gated Molds
Applying a scientific method to what is typically a trial-and-error process. Part 2 of 2.
Read MoreImprove The Cooling Performance Of Your Molds
Need to figure out your mold-cooling energy requirements for the various polymers you run? What about sizing cooling circuits so they provide adequate cooling capacity? Learn the tricks of the trade here.
Read MoreRead Next
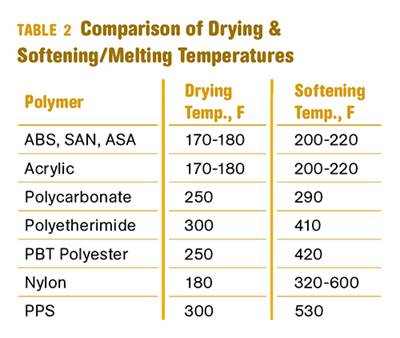
Why (and What) You Need to Dry
Other than polyolefins, almost every other polymer exhibits some level of polarity and therefore can absorb a certain amount of moisture from the atmosphere. Here’s a look at some of these materials, and what needs to be done to dry them.
Read MoreLead the Conversation, Change the Conversation
Coverage of single-use plastics can be both misleading and demoralizing. Here are 10 tips for changing the perception of the plastics industry at your company and in your community.
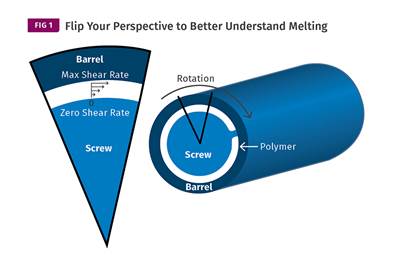
Read MoreUnderstanding Melting in Single-Screw Extruders
You can better visualize the melting process by “flipping” the observation point so that the barrel appears to be turning clockwise around a stationary screw.
Read More