MEDICAL MOLDING: Configure Your Molding Machine Into a ‘Clean Room’
You can meet the stringent requirements of the medical market without having to invest in a full-blown production clean room.
For decades now, clean rooms have been used in plastics processing to produce medical and other high-end ite ms in large volumes and assemble them into finished components. While the standards for clean rooms in injection molding may be roughly the same as those required in other industries, the molding process places a unique set of demands on any part producer that needs to manufacture in a such an environment.
For one thing, due to the crane heights required for installing and removing molds, production clean rooms need a vertical clearance of 16 to 18 ft. Three-axis pick-and-place robotic systems, which enter the clamping unit from above, could require even greater ceiling heights.
Clean rooms are expensive to build. Ones erected for production processes cost much more than those made for assembly only. Clean rooms are also expensive to operate. The systems for circulating the air in the clean room are influenced by the required air changes per hour. So when comparing the height of a clean room for production with one used just for assembly, figure as a rule of thumb that roughly twice the volume of air must be circulated in a manufacturing clean room compared with an assembly clean room of normal height.
Despite these issues, there is a way for molders to meet the stringent requirements of medical and other markets without having to invest in a full-blown clean-room structure.
THE INJECTION PRESS AS A ‘CLEAN ROOM’
The answer is to configure the injection molding machine itself into a “clean room.” While rarely considered, this concept is a production-efficient approach that molders should look into as a way to establish clean-room production at a lower cost. Here, the press simply must be encapsulated at the exposed points where the molded parts could come in contact with the environment. After each cycle, the machine itself can transfer parts to the assembly and packing clean room via a conveyor belt. That way, parts never have to leave the clean environment. This type of clean-room set up is new to North American molders but is already used in Europe.
If the actual clean room is used only for assembly and packaging, it can be built to save money. In fact, not only would it require lower ceilings, but its overall size would be about 25% of the size of a full production clean room. Operational costs would be lower as well.
Clean rooms for injection molding production normally must meet the requirements for Class 7 or 8. According to the European Community (EC) GMP Directive, a Class 7 clean room may contain a maximum of 350,000 particles of 0.5 μm size, and 2000 particles of 5 μm, per cubic meter of air in idle condition. With injection molding machines, this level can be reached by placing a clean-room module above the clamp. This module uses air ionization and a preliminary filter and high-efficiency particulate air (HEPA) filters above clamping units and conveyor belts. As the link between the clean room and the machine, the conveyor belt transfers the molded parts from the machine positioned outside the clean room through an air lock into the clean room, where the final assembly and packing take place.
These machines can be readily certified to the required cleanroom classification. In order to fully exclude biological risks and be able to keep the machines clean, use presses with clamping units in stainless steel with nickel-coated fixed and moving mold platens with covered bores. These are easy to keep clean by following the relevant cleaning instructions
and are insensitive to the aggressive agents commonly used when sterilizing injection molded items.
One requirement is to clean the machine and conveyor belt each day by specially trained personnel using Bacillol, an alcoholbased disinfectant, or similar cleaners. Cleaning can be done fairly quickly and reliably because of the high quality of the surfaces.
Here are some other recommended features of presses for clean-room use:
• All-electric or hybrid machines with direct-drive servo systems and water-cooled drive motors in the clamping area to avoid introducing particulate contamination. This is an advantage over machines with air-cooled motors (using fans) or belt-driven clamping systems. Also, no rotary spindle movements should be performed in the clamping area.
• Active dissipation of waste heat from machines and control units by means of a closed and therefore lowmaintenance, low-wear water circuit. This prevents any turbulent air currents due to fans.
• Hydraulic system for core pulls and its temperature control installed in the machine base and operated with food-grade hydraulic fluid.
• Machine base that is covered and elevated on the injection side, making it easier to clean beneath it.
• Abrasion- and scratch-resistant, light-colored, powder-coated machine surfaces.
• Housing for water manifold and riser tubes.
• FDA/NSF H1-recognized lubricants.
• Special sorter flaps so that falling parts come into contact only with stainless-steel surfaces.
INTEGRATING ROBOTIC SYSTEMS
What do you do when a robotic system needs to be integrated into the production sequence? In this case, the normal positioning above the clamping unit for vertical entry of the robot arm generally is not possible. However, it is feasible to use integrated side-entry robotic systems, as well as six-axis robotic systems. Both can be placed next to machine in a docked clean-room environment. The housings feature separate HEPA filters, which upgrade the environment to the same clean-room level as has been established in production. So, in effect, a clean-room has been established outside the clean room itself, meaning that the parts produced never leave the clean-room environment.
MOLD CHANGE: CLOSE ONE AIR LOCK
When a mold has to be changed, it’s necessary only to close the air lock between the machine and the clean room. The HEPA filter can then be pushed over the clamping unit of the press so that access to the clamping system and mold is free and clear. Before starting production, the entire area must again be manually cleaned. Then the HEPA filter must be moved back over the clamping unit and activated. This ensures that the machine is again suitable for clean-room use.
Before restarting the molding process, it is usually necessary to purge and clean the injection unit. Be aware that the purging sequence causes smoke and exhaust gases. This releases a large volume of dirt particles that must be removed from around the injection unit using vacuum extraction if the machines are to be used for production in the clean room.
Tests at Arburg’s facility in Lossburg, Germany, have shown that particle levels after purging
will exceed the permitted number of particles in a Class 8 clean room by two- to three-fold without extraction. However, if the injection machine is outside the actual clean room, no particles can contaminate the assembly and packaging clean room. This is an elegant solution to this problem, which avoids the need to perform costly extraction around the injection unit.
DECENTRALIZED CLEAN-ROOM PRODUCTION
For production of medical products—from disposable syringes to implants—look for a supplier than can customize your machine as needed and will assume overall responsibility for the planning, implementation, and operation of turnkey systems.
The concept of setting up a production environment outside the actual clean room is very different from the usual one to date. However, it offers so many significant advantages that it should be considered as an alternative by any company looking to further expand its clean-room production. Here are some additional advantages to consider:
• The decentralized concept requires far less clean-room capacity than the classic approach to production inside the clean room, which reduces setup and operational costs.
• Purging and cleaning of the injection units takes place completely outside the clean room. This ensures that there is no negative impact on the actual assembly and packaging situation.
• Only the essential personnel required in the clean room needs to be given access, which reduces the risk of contamination through employees or air movements.
• Because there are fewer persons in the clean room, the manufacturing environment becomes more productive, as less time is required for preparations for entering and leaving the clean room
• Machine operators do not need to follow special dress codes or cleaning procedures.
Related Content
Machine Series Debuts for Chen Hsong
Chen Hsong has three injection molding machines in its booth, including 2-platen, hybrid and a next-generation line with improved platen and toggle design.
Read MoreWord Games: What’s a ‘Hybrid’?
Any molder will tell you there’s a difference in working with electric vs. hydraulic drives. Servohydraulic is still hydraulic; a hybrid machine is something different. Imprecise use of terms causes needless confusion.
Read MoreUpdated Control, Cooling Water Distribution, Electromechanical Ejector and Injection
NPE2024: Boy’s U.S. subsidiary marks its 50th anniversary in Orlando with six machines and U.S. intros for the Procan ALPHA 6 control, and hybrid ejectors and injection units.
Read MoreCustom Injection Molder Plugs into All Electric Machines
Formerly a showroom for early-aughts-era Van Dorn hydraulics, the newest additions to Drummond Industries’ transforming fleet are all-electric Niigata injection molding machines.
Read MoreRead Next
Lead the Conversation, Change the Conversation
Coverage of single-use plastics can be both misleading and demoralizing. Here are 10 tips for changing the perception of the plastics industry at your company and in your community.
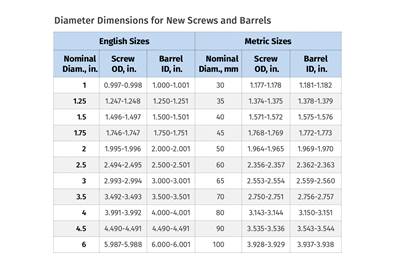
Read MoreTroubleshooting Screw and Barrel Wear in Extrusion
Extruder screws and barrels will wear over time. If you are seeing a reduction in specific rate and higher discharge temperatures, wear is the likely culprit.
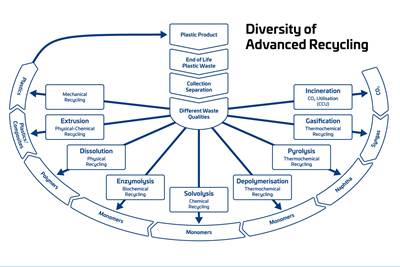
Read MoreAdvanced Recycling: Beyond Pyrolysis
Consumer-product brand owners increasingly see advanced chemical recycling as a necessary complement to mechanical recycling if they are to meet ambitious goals for a circular economy in the next decade. Dozens of technology providers are developing new technologies to overcome the limitations of existing pyrolysis methods and to commercialize various alternative approaches to chemical recycling of plastics.
Read More
















.png;maxWidth=300;quality=90)