So You Want to Be in Medical Plastics? Ask Yourself These Questions First
A medical custom processor cautions those in the field, or wanting to get into it, about regulatory trends that add complexity and risks to an already demanding market sector. Understanding the answers to four key questions can help clarify the issues.
Medical plastics have a reputation for attractive profitability, steady market growth, and being relatively “recession-proof” and largely immune to overseas competition. Altogether, that’s a powerful magnet for a growing number of plastics processors to consider entering this market—or to focus their business more intently on this sector if they are already in it.
But as no rose is without its thorns, participating in medical plastics processing demands a level of sophistication in quality systems, regulatory compliance, materials and design under- standing, and relationships with suppliers and customers that impose significant costs and administrative burdens—both of which are increasing year by year.
That was the message delivered by Robert Schwenker, business manager for Medical Components in the Fluid Systems div. of Saint-Gobain Performance Plastics Corp., headquartered in Solon, Ohio. That company is a full-service custom extruder and injection molder of medical plastics and also formulates some of its own materials. Schwenker spoke to nearly 100 processors attending the most recent Medical Symposium presented by Engel Machinery, Inc. at its headquarters in York, Pa., this past April.
His cautionary message to current or prospective medical processors boiled down to four “Questions to Ask Yourself”:
• Is your quality system keeping up with supply-chain evolution?
• Who is actually making the medical device?
• Have you “thought beyond the print”—in terms of particulates, bioburden, or design controls?
• Are you participating in the supply chain, or just “in” the supply chain?
QUALITY & REGULATORY BURDENS GROWING
Schwenker began his speech by pointing out these regulatory trends in medical manufacturing:
• The annual number of FDA warning letters has increased significantly since 2011, averaging 134 in 2011-2015, up from 83 in 2006-2010.
• Annual FDA inspectional observations are also on an uptrend. From the point of view of medical processors, what’s particularly significant is the rising number of FDA inspections related to product and process control and also design control.
• The 2016 revision to the ISO 13485 medical quality standard for the first time explicitly requires an organization to comply with all applicable regulatory requirements. Before then, the standard did not include a regulatory component.
Schwenker noted that estimates of SG&A (selling, general & administrative) costs related to quality and regulatory systems already amount to somewhere between 6% and 23% and are apt to grow. That’s one reason, in Schwenker’s view, why some estimates of the growth rate in outsourcing of medical-device manufacturing are above 10%/yr: “Not only are medical-device OEMs seeking to harness the manufacturing power of the supply chain, but they are looking to outsource quality and regulatory risk as well.”
At the same time, custom processors face increased quality expectations at the OEM level, said Schwenker. “Fifteen years ago, first-article parts were sufficient for product release, and initial production runs might have been considered ‘provisional.’ Ten years ago, improved measurement systems allowed for more critical dimensions, and validation was typically successful after three production-ready PQs (Performance Qualifications). Five years ago, nearly all new parts required CpKs and IOQs (Installation/Operation Qualification) before undertaking customer-defined validation programs.
“Today, manufacturers have validation programs for equipment and facilities, and defined programs for new-product introduction, all of which are extending lead times. Six months of validation after tool build and testing is not unusual. Also, customers are insisting on supply and quality agreements.”
Schwenker says another problem is that changing a certified medical production process is expensive. “How can a processor afford to implement new technology that could increase quality? He’s stuck. It’s easier to keep a process going the same way than to try to improve it.” The consequence is that processors “need to develop a way to manage change. They need to be proactive, not reactive.”
ARE YOU MAKING A MEDICAL DEVICE—OR A COMPONENT?
That seemingly simple question is getting harder to answer, and the regulatory impact is substantial. The FDA is empowered by statute to regulate “devices” rather than components. But now the FDA interprets “finished devices” as including so-called “accessories.” On Dec. 30, 2016, FDA issued Guidance 1770, which defined a “finished device” (as per 21 CFR820.3) as “any device or accessory to any device that is suitable for use or capable of functioning, whether or not it is packaged, labeled, or sterilized.”
Guidance 1770 further defines an accessory as “A finished device that is intended to support, supplement, and/or augment the performance of one or more parent devices” (see short. ptonline.com/FDA). According to Schwenker, that definition could potentially be extended to include fixtures used in manufacturing devices even though they do not contact the patient at all.
As of October 2012, all contract manufacturers and sterilizers of finished devices must register with FDA. A maker of medical “components” does not have any regulatory obligations—those are the responsibility of the “finished device” maker that buys the components. But FDA’s Quality System Final Rule (61 FR 52609) of Oct. 7, 1996, Guidance 1770 states, “Devices that have been manufactured or assembled, and need only to be sterilized, polished, inspected and tested, or packaged or labeled by a purchaser/manufacturer are clearly not components, but are now in a condition in which they could be used, therefore meeting the definition of ‘finished device.’”
Therefore, Schwenker pointed out, a component that needs only sterilization, polishing, testing, packaging, or labeling in order to be put into use is, in fact, a finished device, and thus imposes all relevant regulatory responsibilities on the producer.
PARTICULATES, BIOBURDEN, DESIGN CONTROL
If a processor can avoid falling under the ever-spreading umbrella of a “device” manufacturer, Schwenker said, “Congratulations, you are a component manufacturer. But you’re not out of the woods.” Three growing trends that affect all medical processors are particulates, bioburden, and design-control methodology. Unless you plan ahead for these concerns, “They are apt to arise only when you get caught,” Schwenker warned.
Since 2012, the FDA has been issuing more specific guidance on particulates. They must be managed at the source, because they are hard to eliminate once present, and any particulates on components can severely impact down-stream production of a finished device.
According to Schwenker, “Typically, component manufacturers try to manage particulates by installing cleanrooms. But a cleanroom without a control and monitoring plan is really just a showpiece.” Required programs include the following:
- Control of raw materials and equipment with wipedown procedures;
- Good cleanroom practices and gowning;
- HVAC control and monitoring and annual certifications;
- Facility sanitization programs;
- Cleanroom environmental monitoring with defined action levels;
- Facility qualification;
- Procedures for startup after a cleanroom breach;
- Validation plans to manage changes.
“The greatest source of particulates is humans,” notes Schwenker. “So we can automate to minimize human interactions. We can contain human particulates with gowns, caps, and gloves. And we can clean off persons entering the clean room in blowoff chambers.”
And what about machinery? He recommends such measures as using all-electric presses, antistatic paint for easily cleanable surfaces, shrouding hoses and lines in plastic, using shields to protect equipment and control airflow, and ensuring controlled airflow through clamp units.
“The bigger question,” he warns, “is how do you know any of this is working?” For that you need careful monitoring of environmental conditions inside the cleanroom.
If particulates are “non-viable” contamination, there’s also the category of “viable” contamination, known as bioburden. The latter includes both live bacteria, or “Colony Forming Units (CFUs)” and cell walls of dead bacteria, which can contain endotoxins that will elicit a biological response in patients. Endotoxins can be removed through washing, but sterilization does not reduce their impact.
Sources of bioburden can include humans, materials, HVAC systems, water and air lines, and cleaning systems. “Just like in your home, without regular monitoring, nature will just take over,” said Schwenker.
The third area of growing concern is design control at the component level. “The new ISO 13485:2016 version requires a more thorough understanding of design control that is more aligned with CFR 820,” noted Schwenker. “The new direction centers around the concept of risk assessment.”
Design control for component manufacturers has two elements:
• “Knowledge of use”—which affects material recommendations;
• “Review for manufacturability”—which concerns tooling and processes.
“While component manufacturers are not responsible for the function of a medical device, their services can include material and manufacturing recommendations,” Schwenker noted. “As supply-chain partners, our ability to recommend materials requires a certain ‘knowledge of use’ of the product.” In addition, it requires the component makers to develop their own supplier-management programs, which can involve materials risk assessment and stability testing, materials compliance data, biocompatibility test data, regulatory monitoring, and agreed-upon change-management procedures.
When it comes to review for manufacturability of tooling and processes, Schwenker said it is crucial to understand potential failure modes and institute appropriate safeguards. “You need to do more work upfront to make sure you are doing it right. The biggest casualty will be time at the front end.”
Schwenker’s conclusion is that “you need to manage beyond the part you make. You need to monitor and stay informed up and down the supply chain to include your material suppliers and the medical-device companies that will use your components. You need to understand material profiles. It takes dialogue.”
Related Content
Collaboration will bring Recycled Plastic to Medical Device Packaging
Agreement between Eastman and Ethicon will put copolyester derived from recycled materials in sterile barrier applications.
Read MoreKrones Acquires Netstal
Krones adds PET preform injection molding to its bottle blowing and filling capabilities, as well as cap molding and expansion into medical, food and other markets.
Read MoreMedical Manufacturer Innovates with Additive Manufacturing and Extrusion Technology Hubs
Spectrum Plastics Group offers customers two technology hubs — one for extrusion, the other for additive manufacturing — to help bring ground-breaking products to market faster.
Read MoreNew Cap for Child-Resistant Pill Bottles Is Senior-Friendly & Saves Resin
Exclusive lightweight cap design can be removed simply pushing down on the center. No gripping or twisting needed.
Read MoreRead Next
What It Takes (And Doesn’t Take) To Be a Medical Molder
Medical molders absolutely must have a clean room and be ISO certified, right? ‘Wrong,’ says Extreme Molding’s Joanne Moon.
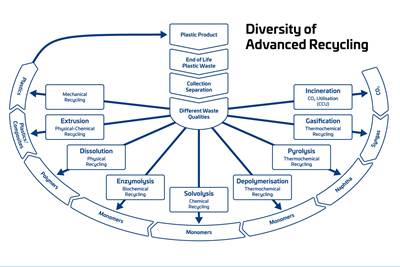
Read MoreAdvanced Recycling: Beyond Pyrolysis
Consumer-product brand owners increasingly see advanced chemical recycling as a necessary complement to mechanical recycling if they are to meet ambitious goals for a circular economy in the next decade. Dozens of technology providers are developing new technologies to overcome the limitations of existing pyrolysis methods and to commercialize various alternative approaches to chemical recycling of plastics.
Read MorePeople 4.0 – How to Get Buy-In from Your Staff for Industry 4.0 Systems
Implementing a production monitoring system as the foundation of a ‘smart factory’ is about integrating people with new technology as much as it is about integrating machines and computers. Here are tips from a company that has gone through the process.
Read More