Understanding the ‘Science’ of Color
And as with all sciences, there are fundamentals that must be considered to do color right. Here’s a helpful start.

Molded-in color is an advantage of polymer materials. Photo: Avient
When enumerating the advantages of using plastics over other materials such as metals, ceramics, and wood, one of the items that inevitably comes up is molded-in color. Even in industries like automotive that still rely on painting plastics, the long-term objective is to achieve the desired finish and durability with molded-in color. The plastics industry has touted this advantage for such a long time that we frequently do not consider the intricacies and potential problems that can arise when incorporating color into a polymer. But there is a science to this process; and as with all sciences, there are fundamentals that must be observed if things are to work properly. In this article we will briefly review these fundamentals and discuss the things that we observe when we fail to adhere to them.
Compatibility
There are several key items that are essential in developing a colorant package for any polymer. The first of these is the avoidance of any chemical incompatibility between the chemistry of the polymer and the chemistry of the colorant system. Chemical reactions proceed more rapidly at higher temperatures, and melt processing involves significantly elevated temperatures and large inputs of mechanical energy. Therefore, any chemical interactions that may occur between a polymer and a colorant system will happen very rapidly during the injection molding process.
A comprehensive treatment of the negative chemical interactions are too numerous to catalog in an article of this length. However, a couple of examples are worth considering. The literature is full of papers on the subject of negative interactions between PC and TiO2, the primary ingredient in white colorants. However, as research progressed it was discovered that TiO2 was not the problem. Titanium dioxide is not a synthetic; it is a mineral that is pulled out of the ground along with a lot of other constituents. Among these are compounds such as hydroxides of aluminum and potassium. These are bases and polycarbonate does not do well in environments where bases are present. So imagine the consequences of exposing polycarbonate to this type of chemical at 600 F (315 C). Degradation will occur rapidly. Today suppliers of white pigments using TiO2 know that the pigment must be treated to neutralize these basic constituents.
Sometimes the interaction is not between the colorant and the base polymer but with a minor constituent in the material. When the initial efforts to remove heavy metals such as cadmium and lead from pigments first began in the early 1990s, the conversion for some materials was quite seamless while in others it was a difficult process. Nylon was an example that required multiple iterations before we got it right. But interestingly, the initial tests on cadmium-free red colorants with nylon worked quite well. It was not until impact-modified grades of nylon were tried with the new pigments that problems with ductility became apparent. The same colorant that worked well for a general-purpose glass-filled nylon was not suitable for the same material formulation with impact modification.
Thermal Stability
The next consideration in selecting a colorant system is the thermal stability of the colorant system. The colorant chemistry must be capable of surviving the temperatures at which the material must be processed. Therefore, a colorant that may be used for polyethylene or polypropylene, where processing temperatures will be relatively low, may be completely unsuitable for a material such as polycarbonate or polysulfone, even if it is acceptable strictly on the basis of chemical compatibility. Going back to the conversion of red nylon from cadmium-based to cadmium-free colorants, the initial attempts at producing cadmium-free colorant systems often resulted in parts that were much darker in color than was desired. The color shift was produced by the elevated temperatures and, in some cases, by the high shear rates associated with molding large parts with long and complex flow paths.
Colorant Amount
Good property retention is also a function of the amount of colorant that is incorporated into the polymer. Colorants are contaminants, but they are contaminants that we tolerate because they help us achieve a desired effect. But for each combination of polymer and color there is a limit to how much color can be added before the properties of the material are negatively affected. Typically, the first short-term property to exhibit a decline is ductility. Additions of 1-2% of a colorant are usually harmless as long as the chemical compatibility issues mentioned above are accounted for.
However, as colorant levels rise the chances are greater that impact performance will decline. Often the focus of those developing a new color is strictly on aesthetics. But in some cases achieving a desired color match can involve adding relatively large amounts of color to the base resin, shifting the properties considerably.
For example, I once worked with a customer to develop a white polyetherimide (PEI). Natural PEI is transparent but it is also quite dark in color. Making it white can require a lot of TiO2. In this case, by the time the desired appearance was attained, the TiO2 content was 9%. But the client expected the properties to be the same as that of the natural resin. If I were to tell end users that an unfilled grade of material and a grade that contained 10% of a filler would have the same properties, most of them would be justifiably skeptical. But no one considered the addition of a comparable amount of an inorganic colorant to be problematic.
Often, the threshold levels are much lower and are not discovered until something goes wrong. About 10 years ago, one of my clients suddenly encountered a problem with notch sensitivity in a white PC. Toughness was only reduced noticeably in areas of the part where sharp corners existed and it appeared to be related to a particular lot of material.
This material was fully compounded, and the assumption is often made that formulations are very consistent when a compounder puts the color in as opposed to when it is added as a concentrate at the molding machine. But in comparing the pigment loading of a lot of raw material that produced good parts to one that produced brittle parts, we found that the colorant content had increased from 2% to nearly 4%. This prompted us to perform some experiments where we made small samples with pigment loadings that varied from a low of 1% to a high of 4%. We found that the notch sensitivity began to appear when the colorant content reached 2.5% and became progressively worse as the amount increased from there.
There was an interesting wrinkle to this problem. In reviewing the compounder’s records we found that their control over pigment content had never been very good, and yet over the course of a very long history for the product this problem with brittle performance had never been observed. The reason was that the base PC had changed about 12 months earlier from a V-2 flammability-rated material to a V-0 rated material. This meant that the new material contained a slightly higher concentration of a flame retardant. When we repeated the experiment on the notch sensitivity-color loading relationship with the original material we found that there was no sensitivity to the amount of color. The properties were the same at 4% as they were at 1% and everywhere else in between. This showed that it was not as simple as an interaction between the colorant and the polycarbonate. The flame retardant was part of the picture. This points out the importance of considering potential changes due to additives.
Industry Changes
Molecular weight is also a factor that influences the behavior of a polymer when certain colors are added. For those of us who have been around the industry for a while, we remember the days when the major material suppliers considered the production of custom colors in their resins as an important core competency. I recall visiting an ABS plant operated by Borg-Warner (which subsequently was bought by GE Plastics, which then became SABIC Innovative Plastics, etc.) The person giving us the plant tour proudly told us that they had almost 40,000 different colors on file and that of these about 6000 were active. Not only would they make just about any color you wanted, they would do so at minimum levels that would be considered ludicrously small by today’s standards.
Even companies that did not go this deeply into the coloring business still had 10-12 “standard colors” that they would make in most or all of their base resins. I always found it interesting that in Lexan PC, GE would make both transparent and opaque colors in their higher-molecular-weight grades such as Lexan 101 and 141. But they would only sell transparent colors in the so-called high-flow grades. The reason was simple: Transparent colors can be made with dyes that are potentially less intrusive to the polymer structure than pigments. Pigments consist of particles, and the size of those particles is important to the performance of the material. If they are too small or too large they can present a problem. The properties of higher-molecular-weight PCs were not significantly affected by the opaque colorants, but the lower-molecular-weight materials were.
Later, as GE expanded its color offerings, the firm eased these restrictions. But they also began to publish data sheets that made a distinction between the impact strength of the natural material and the opaque colored materials. Some of the information in those data sheets was cause for concern. For example, three grades of base resin with melt-flow rates of 7, 15, and 25 g/10 min all had notched Izod impact values of 14-16 ft-lb/in. in natural and in transparent colors. But in the opaque colors, the Izod impact values for the 7-MFR and 15-MFR materials remained at the same levels while the properties for the high-flow grade were given as a range. And the range was 2 to 14 ft-lb/in. The actual results depended on which colorant was being added.
Now that the primary material suppliers have largely stepped away from the coloring business, the expertise has been taken up by the custom compounding community. It is now their job to be aware of these considerations of pigment loading, chemical compatibility, and molecular weight. As can be expected, this expertise is not homogeneously distributed across the industry.
Crystallinity
One effect that can be difficult to anticipate is the influence of a colorant on the manner in which crystallinity develops in a semi-crystalline polymer. Many colorants are natural nucleating agents in a material such as PP. Nucleation changes the crystal structure of the material and at the same time changes mechanical performance, shrinkage, and cycle time. Nucleated materials cycle faster and shrink less than their non-nucleated counterparts. But they also tend to be less impact resistant. If you have ever had the task of molding close-tolerance parts in multiple colors of a given semi-crystalline polymer such as PP or acetal (POM), you have probably noticed that you obtain different dimensions in different colors and may have to make process changes to bring the parts back to print. This is not your imagination and it is not a case of molding gremlins, it is a manifestation of the manner in which constituents in the colorants change the crystal structure of the base polymer.
Mixing
The manner in which the color is incorporated into the base resin is also important. Fully compounded color is often preferred because it bypasses problems with color uniformity that often occur when color is added as a minor second ingredient in the form of a pellet concentrate, a dry color, or a liquid. But the economics of coloring at the point where the part is being manufactured are much more favorable for a lot of reasons. These include cost per pound, lead times, and the ability to control inventory and avoid expensive obsolescence. If you purchase 5000 lb of avocado green ABS, you are betting on the continued popularity of that color in the product you are making. Sometimes that works out, sometimes it doesn’t. If the coloring is being done only at the point when the mold is put into the press, the natural material is not committed to any particular color until it is time to make parts.
But now the color represents a separate ingredient and there are considerations in specification that need to be managed. In a color concentrate, part of the pellet is the colorant and part of it is polymer, a so-called carrier resin. For best results the carrier resin must be compatible with the base resin into which it is being placed. This means that if you are coloring PC the color concentrate pellets should also be made of PC, not PE or EVA copolymer. There is some latitude. Nylon 66 can be colored with a concentrate based on nylon 6. Polypropylene and polyethylene are reasonably compatible. SAN can be used in a concentrate designed for ABS, but PS cannot.
But here is the important message. There is no such thing as a universal carrier. Anyone who has done polymer blending knows that very few polymers mix well with other polymers. Often the calculation is that since the color concentrate is only being added at two to four parts per hundred the contamination can be tolerated. But if the control over the proper mix ratio is not optimal, then the amount of this contaminant also fluctuates and problems can ensue. Sometimes a primary material supplier will provide what is referred to as a salt-and-pepper blend—natural pellets and color concentrate both made by the material supplier and mechanically mixed at the correct ratio before packaging. It would be nice to assume that at least these products are made with appropriate attention to material compatibility. Unfortunately, this is not always the case.
In my manufacturing days, we used to make a product in an impact-modified, mineral-filled nylon 66 in both natural and black. The raw material was made by a major supplier and they used a salt-and-pepper mixture for the black. We noticed that while the natural material ran very well, the black parts exhibited a lot of cosmetic issues and the process was very inconsistent because the screw had a tendency to slip during recovery. We finally analyzed the black concentrate and found that the carrier resin was EVA copolymer. At the temperatures at which filled nylon 66 is processed, EVA begins to decompose and turn to wax, thus creating the problems we were seeing. When we asked the supplier to replace the colorant with one based on nylon 6, they refused, citing cost. We finally became tired of the high scrap rates and constant babysitting of the process and began purchasing only natural. We added our own nylon-based concentrate purchased from our concentrate supplier.
Melt Viscosity
The other big mistake that is made with color concentrates has to do with the molecular weight of the carrier resin. Even when the carrier is compatible with the base resin, often we find that the melt-flow rate of the carrier resin is eight to 10 times higher than that of the base material. It is believed that this is required to promote good mixing. Not only does this practice introduce a low-molecular-weight constituent into the final part, reducing properties as well as the processing window, but the principle that a much lower viscosity for the carrier resin improves mixing is simply wrong. Dramatically different viscosities introduced into the molding machine barrel tend to segregate rather than mix.
You can perform a simple experiment to illustrate this. Take refrigerated mayonnaise and ketchup, which are typically of similar viscosities, and mix them in a bowl. Now perform the same task but replace the ketchup with tomato juice. You will observe that it takes a lot more stirring to evenly incorporate the low-viscosity tomato juice than it does the higher-viscosity ketchup. A small difference in viscosity is certainly helpful, but a large one is not and actually does harm to the final product.
This is a very quick review of an activity that we tend to take for granted, incorporating color into our raw materials. There is a lot to think about and the discipline of coloring, as with most important activities in our industry, is more complex than we think. So the next time you are in a development meeting and someone says they want the product to be blue or red, put this on the list of items that need to be addressed just as carefully as the rest of the material selection process.
ABOUT THE AUTHOR
Mike Sepe is an independent, global materials and processing consultant whose company, Michael P. Sepe, LLC, is based in Sedona, Ariz. He has more than 35 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact:
(928) 203-0408 • mike@thematerialanalyst.com.
Related Content
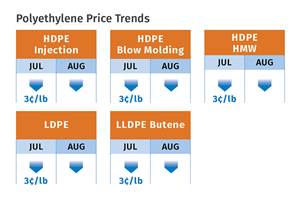
Prices of All Five Commodity Resins Drop
Factors include slowed demand, more than ample supplier inventories, and lower feedstock costs.
Read MoreResin Prices Still Dropping
This downward trajectory is expected to continue, primarily due to slowed demand, lower feedstock costs and adequate-to-ample supplies.
Read MoreThe Effects of Stress on Polymers
Previously we have discussed the effects of temperature and time on the long-term behavior of polymers. Now let's take a look at stress.
Read MoreCommodity Resin Prices Flat to Lower
Major price correction looms for PP, and lower prices are projected for PE, PS, PVC and PET.
Read MoreRead Next
Troubleshooting Screw and Barrel Wear in Extrusion
Extruder screws and barrels will wear over time. If you are seeing a reduction in specific rate and higher discharge temperatures, wear is the likely culprit.
Read MoreProcessor Turns to AI to Help Keep Machines Humming
At captive processor McConkey, a new generation of artificial intelligence models, highlighted by ChatGPT, is helping it wade through the shortage of skilled labor and keep its production lines churning out good parts.
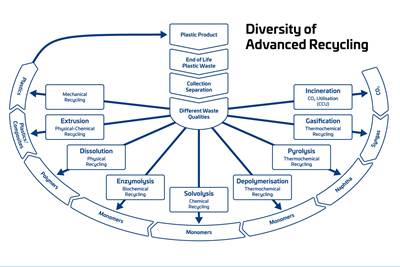
Read MoreAdvanced Recycling: Beyond Pyrolysis
Consumer-product brand owners increasingly see advanced chemical recycling as a necessary complement to mechanical recycling if they are to meet ambitious goals for a circular economy in the next decade. Dozens of technology providers are developing new technologies to overcome the limitations of existing pyrolysis methods and to commercialize various alternative approaches to chemical recycling of plastics.
Read More
.jpg;width=70;height=70;mode=crop)











.png;maxWidth=300;quality=90)