Why (and What) You Need to Dry
Other than polyolefins, almost every other polymer exhibits some level of polarity and therefore can absorb a certain amount of moisture from the atmosphere. Here’s a look at some of these materials, and what needs to be done to dry them.
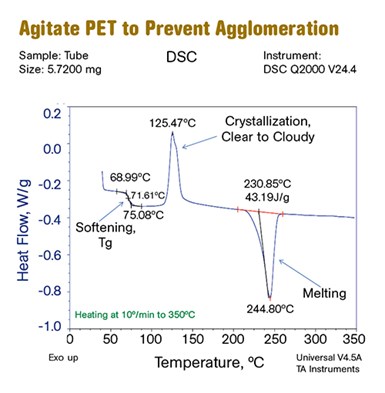
A DSC scan of PET bottle resin reveals that it goes through a glass transition, evident as a step transition in the thermogram, at around 70-75 C. At this point it will soften. When it reaches a temperature of about 110 C the polymer begins to crystallize. At 245 C these newly formed crystals melt. If the amorphous form of PET were heated from room temperature to the required drying temperature, it would agglomerate into one large mass and would then crystallize in that same large mass. To prevent this, PET must be constantly agitated while it is heated through its glass-transition temperature and its solid-state recrystallization. PLA exhibits similar behavior.
In most plastics processing plants, resin dryers are a standard piece of equipment. The notable exception to this would be facilities that exclusively process polyolefins such as polyethylene and polypropylene. These are among the few polymer families that do not require drying because these material families are not hygroscopic. Materials like polyethylene are non-polar, while water is a highly polar substance that acts like a small magnet with a negatively charged and a positively charged end. Water has no affinity for non-polar polymers. Exposing polyethylene or polypropylene to water is like placing oil in water. The two substances separate immediately because they have nothing in common chemically.
But almost every other commercial polymer exhibits some level of polarity and therefore is capable of absorbing a certain amount of moisture from the atmosphere. The amount of moisture that any given polymer can absorb depends upon the chemistry of the polymer and the atmospheric conditions to which it is exposed. Polymers such as blends of PPE and HIPS are only slightly polar and even at saturation can only hold 0.07% moisture. At the other end of the spectrum is nylon, a highly polar polymer that when taken to the point of saturation can absorb 8-9% water or over 100 times as much as PPE/HIPS compounds.
However, even moisture levels of 0.07% can cause cosmetic problems on a part surface if the material is exposed to the temperatures of melt processing. The resulting defect, known as splay or silver streaking, is an indicator that the material being molded contains too much moisture. Drying is a necessary preparative step to prevent this defect.
While many materials are dried solely to optimize surface appearance, some polymers undergo more significant changes if they are processed in the presence of too much moisture. These materials actually enter into a chemical reaction with the moisture—called hydrolysis. This mechanism breaks the covalent bonds in the polymer chain, reducing the molecular weight of the polymer and potentially resulting in a significant reduction in mechanical properties.
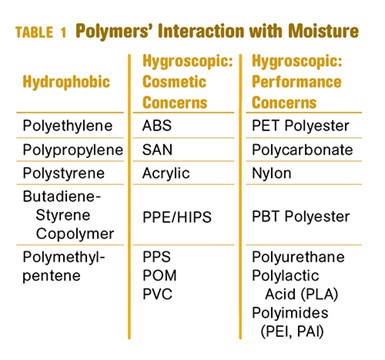
Table 1 classifies polymers according to their behavior in relation to water. Hydrophobic materials cannot absorb any significant amount of moisture. Any moisture that could be present in these materials will remain on the surface of the pellets and seldom rises to a level greater than 0.01%, not enough to cause any cosmetic or structural problems.
On rare occasions there are reports of cosmetic defects in parts molded in polyethylene or polypropylene that are traced to excess surface moisture deposited due to rapid changes in the temperature and humidity of the plant. But these are usually easily rectified by simply allowing the raw material to come to equilibrium with the surrounding atmosphere so that the surface moisture can evaporate.
The Two Types of Hygroscopic Materials
Hygroscopic materials break out into two subsets. One group experiences only cosmetic problems if not properly dried. Members of the other group suffer irreversible structural damage due to hydrolysis. Table 1 does not present an exhaustive list, but it includes many of the commonly used polymer families. It is also important to emphasize that blends need to be treated with the care required of the most sensitive polymer in the blend.
For example, a polycarbonate/ABS blend needs to be treated with the same care as polycarbonate. Sometimes seemingly subtle changes in formulation can change the importance of drying requirements. For example, it is generally considered unnecessary to dry acetals (POM); however drying is recommended for impact-modified POM because the impact modifier is typically polyurethane, which is subject to hydrolysis.
There are other important differences between the hygroscopic materials that exhibit only cosmetic defects and those that can degrade. With the exception of nylon, all the materials in the column with performance concerns must be dried to lower moisture contents than those materials with only cosmetic concerns.
Most of the polymers in the “Cosmetic Concerns” column can be successfully processed at moisture contents of 0.05-0.10%. However, the majority of the materials in the “Performance Concerns” column should not be processed at moisture levels higher than 0.02% and some of these materials achieve optimum properties when dried to levelsas low as 0.005%. The optimum moisture level depends not only on the polymer but also on other processing parameters such as melt temperature and residence time in the molten state.
The type of process may also have an influence: profile extrusion, for example, may require a lower moisture content than injection molding. Secondary operations such as plating or ultrasonic welding may also dictate a need for tighter control of the material’s moisture content.
Proper drying requires attention to detail in the following areas: temperature and moisture content (dew point) of the inlet air, volumetric flow rate of the air across the pellets, time the material is in the drying hopper, and temperature of the return air coming back from the hopper to the drying unit. There are other areas of concern related to the design and maintenance of the system. This also assumes that a desiccant dryer is being used.
There are other methods, like vacuum and compressed-air drying. Other technologies such as infrared and radio frequency have been explored, but most commercial systems today still utilize some variant of desiccant technology. However, many systems still use hot air without the aid of a desiccant. While these systems are less complex, they lack the ability to consistently provide dry air to the material in the hopper.
Most material suppliers make specific recommendations for drying times, temperatures, and the dew point of the air being supplied to the drying hopper. Commonly recommended dew points fall between -20 and -40 F. These levels can be achieved only by passing the inlet air over or through a drying medium (desiccant or membrane filter). Otherwise, the dew point of the air will be whatever it is in the plant. On winter days in cold climates this number might be as low as 0 F, but on hot, humid days it can rise to 70-80 F.
At these conditions it is not possible to bring the moisture content of a raw material down to the levels required for the “Performance Concerns” group in Table 1, regardless of how long the drying process may be. It should be understood that much of the plastics material processed in Asia is not dried in desiccant systems and a significant amount of that material falls into the “Performance Concerns” category.
The conditions for effective drying are dictated to a significant extent by the polymer being processed. An elevated drying temperature is desirable since it increases the rate of moisture removal. However, the drying temperature must remain below the softening or melting point of the material. This is more likely to be a problem in amorphous materials, where the recommended inlet air temperature may be reasonably close to the softening point, or glass transition temperature, of the material.
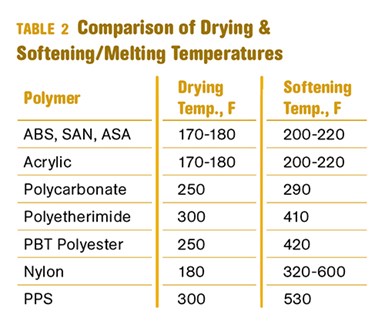
Table 2 compares recommended drying temperatures with the softening or melting points of some commonly used polymers. It can be seen that for amorphous materials like ABS, polycarbonate, and acrylic, the difference between the drying temperature and softening temperature is relatively small. Anyone who has spent time in a molding plant has likely experienced the unpleasant consequences of a temperature override in a dryer when working with these materials.
The rules become a little more complex for polymer families where softening temperatures vary as a function of composition. One example is PPE/HIPS alloys. Grades with a high polystyrene content may have softening temperatures as low as 190-200 F and must be handled more carefully than high-PPE grades where the softening point may be as high as 330-340 F. Similarly, elastomers such as TPUs will exhibit a range of softening temperatures related to the Shore hardness of the grades.
It may appear from the table that semi-crystalline materials like nylon and PBT polyester offer an opportunity to use much higher temperatures than are recommended. However, with these materials the potential exists for oxidation, a chemical reaction that can damage the polymer irreparably before it ever reaches the feed throat of the machine. This is particularly a concern for nylons.
PET, PLA: Special Step Required
There are two materials in Table 1 where special steps are required during the drying process. These are PET polyester and PLA biopolymer (also a polyester). These materials go through a change from amorphous to semi-crystalline before ultimately melting. Figure 1 shows a DSC (differential scanning calorimetry) scan for the PET used to make beverage bottles. It goes through a glass transition, which appears as a step transition in the thermogram, at approximately 70-75 C (160-170 F). At this point it will soften.
However, when it reaches a temperature of about 110 C (230 F) the polymer begins to crystallize. If the sample could be viewed during this process you would see the material turn from transparent to cloudy and then opaque. This process is complete by the time the temperature reaches 140-150 C (285-300 F) depending upon the grade of material.
Finally, at a temperature of 245 C (473 F) these newly formed crystals melt. PET requires a drying temperature range of 135-165 C (275-330 F) and I have seen temperatures as high as 180 C (356 F) utilized in an effort to optimize performance. If the amorphous form of PET were heated from room temperature to the required drying temperature, it would agglomerate into one large mass and would then crystallize in that same large mass at temperatures high enough to ensure effective moisture removal.
To prevent this, PET must be constantly agitated while it is heated through its glass transition temperature and its solid-state recrystallization so that it can then be heated to the appropriate drying temperatures without agglomeration. PLA exhibits essentially the same behavior, although the specific key temperatures are somewhat lower than they are for PET. And the stakes are very high for both polymers. Moisture levels as low as 50 ppm may be required in order to successfully process the material into product that has the desired properties.
How to Measure Moisture Content?
This brings us to discussion of the real object of resin drying: moisture removal. There are a lot of parameters in the drying process that are monitored, but the actual moisture content of the dried resin entering downstream processing is really the only thing that matters. Many material suppliers provide guidelines for the moisture content that their resins must have to ensure good property retention. The problem for the industry is that very few processors measure moisture content.
A majority of processors simply follow drying guidelines or their best interpretation of them and assume that this is sufficient. Many processors incorrectly believe that if a material is processed with excessive moisture the parts will exhibit cosmetic defects.
A small percentage of processors employ a device that measures moisture content as a loss in total weight of a sample heated to a particular temperature for a prescribed time period. Unfortunately, these instruments measure all of the volatiles that evolve from the sample. They cannot distinguish between moisture and all of the other volatile constituents that may be in the material. A much smaller group of processors use an instrument that is sensor based and therefore is moisture specific. This is an appropriate strategy and results in processes that are much more reliable and less likely to produce parts with reduced mechanical performance.
But the best approach involves making accurate moisture measurements in the drying hopper. This has become possible within the last two to three years using measurements of dielectric properties in real time. There is a well-defined relationship between the moisture content of a polymer sample and these dielectric properties. This bypasses the need for off-line measurements conducted in the laboratory and allows for continuous real-time documentation of the only parameter that matters. In addition, the monitor can be interfaced with the dryer controls to regulate the drying conditions. This potentially saves energy and can prevent oxidation of the polymer and corresponding damage to the additives.
We can hope that this is the new face of resin drying. While no systematic studies have been performed to assess the lost time and money associated with substandard drying practices, it would be reasonable to estimate that it totals in the billions of dollars annually worldwide.
Improvement begins with an awareness of the shortcomings of current methods and practices. Once they are understood, greater knowledge of the myths and science associated with resin drying can be gained and many of the product failures that are experienced every year can become a thing of the past.
Related Content
New Technology Enables ‘Smart Drying’ Based on Resin Moisture
The ‘DryerGenie’ marries drying technology and input moisture measurement with a goal to putting an end to drying based on time.
Read MoreCaptive Molder Beefs Up Auxiliaries to Boost Quality, Consistency
SeeScan adds conveying, drying, feeding and chilling technologies to improve quality — and enhance employee safety — in production of its underground/underwater inspection systems.
Read MoreA Cost Saving Modular Approach to Resin Drying Automation
Whether implementing a moisture-sensing closed-loop system for a single dryer, or automating an entire plant, technology is available to take the guesswork and worry out of resin drying. Using a modular approach allows processors to start simple and build more capabilities over time.
Read MoreRead Next
Follow These Seven Management Tips for More Consistent Resin Drying
Equipment and advice sure help, but ultimately, good management makes the difference.
Read MoreUse a DOE to Improve Consistency of Your Resin-Drying Process
Conducting a drying design of experiments (DOE) will ensure your polymer is properly dried every time. Here’s how to do it.
Read MoreFour New Developments in Plastics Drying
These four short videos provide a snapshot of what’s going on in plastics resin drying technology. Yes, there’s Industry 4.0, but also lots more to bring higher quality and efficiency to your injection molding and extrusion processes.
Read More.jpg;width=70;height=70;mode=crop)