The Importance of Oxidative Stability in Polyolefins, Part 3
Tools and methods for determining oxidative stability can be useful, but even more useful if constructed to yield the information you really need.
One of the problems that inevitably develops when an analytical technique is formalized into a standard is that the methodology outlined in the standard is often narrowly defined. This leads to the impression that the tests must follow the precise outline in the standard in order for the results to be valid. Often, variations on the standard that could yield useful information are not explored.
The oxidative stability test that we have been discussing typically calls for a test temperature of 200 C (392 F). As we have already mentioned, if this temperature is employed for PE or PP, the sample will be in the molten state when the air or oxygen is introduced. The higher surface-to-volume ratio of the molten sample will result in a shorter time to the onset of oxidation than if the sample was in the solid state. Consequently, while the results obtained at 200 C may give some insight into the stability of the material during melt processing, the results do not provide useful information regarding the oxidative stability of the polymer in a molded part.
To achieve this, we need to perform tests at a temperature below the melting point of the polymer under consideration. For most PPs the crystalline melting point as defined by the minimum point in the DSC (differential scanning calorimetry) endotherm, which is approximately 165 C (329 F). However, a closer examination of this endotherm shows that the onset of crystal melting occurs near 130 C (266 F). For random copolymers this temperature range typically shifts to a point 15 C (27 F) lower, and for polyethylenes it declines even more. Therefore, if the test is to be considered useful for determining oxidative stability in the solid state, the test temperature must be reduced.
The test can be even more useful if conducted at multiple temperatures. The kinetics of the oxidation process in polymers follow a general pattern that is often stated as a rule of thumb: For every change of 10 C (18 F) in the application temperature, the reaction rate changes by a factor of 2. Higher temperatures accelerate the reaction to this degree, while lower temperatures slow things down by the same factor. This represents an Arrhenius function, so the relationship is exponential. Therefore, if the temperature change is 30° C (54° F), the reaction rate changes by a factor of 23 or 8.
But the acceleration factor, or aging factor as it is sometimes called, is not 2 for all materials. It can vary slightly, typically between 1.7 and 2.5. This may seem trivial, but when accelerated tests are performed to monitor oxidative stability, they are typically run at a relatively high temperature and the result is then extrapolated to a lower temperature based on this acceleration factor. Consider an aging test conducted at 150 C (302 F) for a PP that results in failure of the material in 15 days. If this result is extrapolated to an application temperature of 90 C (194 F) using an acceleration factor of 2, the time to failure would be extended by a factor of 26 or 64, giving a prediction of 960 days or about 32 months. But if a factor of 2.2 is used, the extension would be 2.26 or 113.4, giving an anticipated time to failure of 1700 days or 77% longer.
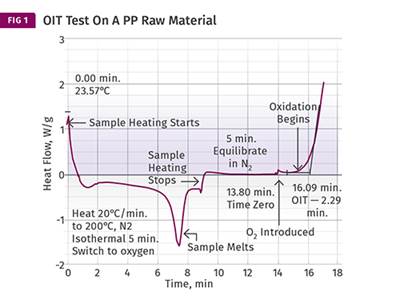
It is useful, therefore, to determine this aging factor more precisely if we are to use it to make these types of predictions. This can be done in a relatively short time frame by performing the oxidation-induction time (OIT) test at multiple temperatures so that the slope of the function that relates time to oxidation as a function of temperature can be determined. The accompanying table shows the results for tests performed at three temperatures for a PP to determine the oxidation induction time at each temperature.
When these results are plotted as the log of the OIT as a function of temperature (see accompanying graph), the data points give reasonably good agreement with a straight line that has a slope of 2.43. This is the acceleration factor that can then be used to make predictions about stability at lower temperatures where actual test times would be very long.
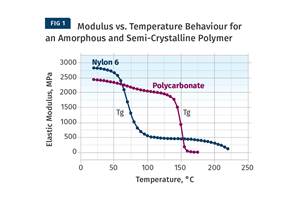
The assumption here is that this slope will be applicable across a wide range of temperatures. We can expect that if we were to raise the test temperature in 10°C increments, when we reached the crystalline melting point the slope would change. We can also anticipate that the slope may change with declining temperature when the material passes through its glass-transition temperature (Tg). However, the Tg for PPs is typically subambient and it is unlikely that we will be concerned about oxidative degradation at subambient conditions. We are more likely to have other problems in this temperature range, such as reduced impact resistance.
Even with such an extended treatment of oxidative stability, we still have the problem of correlating the times associated with these tests to the real-world behavior of a molded product. There are a number of factors to be concerned with.
First, the OIT tests are often conducted in pure oxygen; in the real world the atmosphere is only 21% oxygen. Second, even when our test samples remain in the solid state it will be difficult to replicate the surface-to-volume ratio of a real part with that of the DSC samples. And while the tests monitor the time required to oxidize the sample completely, in a real application, oxidation does not need to penetrate through the entire part thickness to cause problems with performance. Third, in the real world, influences other than temperature can influence the oxidative stability of a material.
For example, PE and PP are frequently used in applications that handle water. Chlorine in the water acts as an oxidizer that can accelerate degradation of the material even in the absence of elevated temperatures. In addition, chloramines that form in water systems have a similar deleterious effect on the long-term performance of these materials. Water can also extract antioxidants from the material, leading to a shorter time to failure than if the part were used in a dry environment. This has been recognized as a potential problem and additives have been developed that resist extraction. It is important to consider the full range of application conditions when evaluating the potential for oxidation and formulating a stabilizer package.
Nevertheless, understanding the tools that are available for evaluating oxidative stability can be very useful, at least as a tool for making relative comparisons. This is especially important when comparing lot-to-lot consistency of a given material or comparing raw material to a molded part. The fact that the method has been around for a long time does not make it less valid, as long as we understand why we are using it and construct a method that is pertinent to the information we are seeking.
ABOUT THE AUTHOR
Mike Sepe is an independent, global materials and processing consultant whose company, Michael P. Sepe, LLC, is based in Sedona, Ariz. He has 40 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 mike@thematerialanalyst.com.
Related Content
The Effects of Temperature
The polymers we work with follow the same principles as the body: the hotter the environment becomes, the less performance we can expect.
Read MorePolyethylene Fundamentals – Part 4: Failed HDPE Case Study
Injection molders of small fuel tanks learned the hard way that a very small difference in density — 0.6% — could make a large difference in PE stress-crack resistance.
Read MoreThe Effects of Stress on Polymers
Previously we have discussed the effects of temperature and time on the long-term behavior of polymers. Now let's take a look at stress.
Read MoreThe Fundamentals of Polyethylene – Part 1: The Basics
You would think we’d know all there is to know about a material that was commercialized 80 years ago. Not so for polyethylene. Let’s start by brushing up on the basics.
Read MoreRead Next
MATERIALS: The Importance of Oxidative Stability in Polyolefins
Because oxidation is a process that causes materials to deteriorate over time, its effects or potential for those effects are not always apparent when new products are tested.
Read MoreThe Importance of Oxidative Stability In Polyolefins, Part 2
The DSC test can do a reasonably good job of capturing the comparative behavior of materials that use similar antioxidant chemistries.
Read MoreWhy (and What) You Need to Dry
Other than polyolefins, almost every other polymer exhibits some level of polarity and therefore can absorb a certain amount of moisture from the atmosphere. Here’s a look at some of these materials, and what needs to be done to dry them.
Read More
.jpg;width=70;height=70;mode=crop)







.png;maxWidth=300;quality=90)