When It Comes to Nylon, Don’t Do the Math
Materials Know How
Chemistry is seldom as simple as it looks. Polymer chemistry takes the complexity up a notch. Nylon chemistry is about much more than doing the math.
Most people do not have fond memories of their formal education in chemistry. Other than the occasional story about crafting something in the lab that frothed, foamed, or exploded, bringing up chemistry class elicits only negative memories of endless battles with structures and reaction mechanisms. This is particularly true of organic chemistry, which is the basis for polymer chemistry. So it is perhaps not surprising that the working knowledge in the plastics industry of what is really going on with our materials is somewhat hazy at best.
This was demonstrated to me once again in a conversation I had just this week with one of my lab colleagues. He had analyzed a nylon 6/6 material for a client and had found the presence of nylon 6. This is not that unusual. Nylon 6 can be grafted onto the backbone of the nylon 6/6 molecule to produce a copolymer that processes at a lower temperature and more easily achieves a resin-rich finish in parts molded of highly reinforced grades.
More commonly, suppliers simply blend the two polymer types to create a balance of properties that falls somewhere between those of the two base resins. In the case of the copolymer, it is not easy to tell that the nylon 6 is present. The material presents a single melting point that falls just about halfway between those of the nylon 6 and nylon 6/6. In the blends, it is a little easier to tell that something has changed because the melting points for the two polymers will appear as separate events when the material is tested by differential scanning calorimetry (DSC). But when my colleague’s client was told of the presence of nylon 6 in the compound they had believed was pure nylon 6/6, someone informed them that this was because the material was degraded and that nylon 6 forms when nylon 6/6 degrades.
This is so far from the real story that it is the organic chemistry equivalent of telling someone that the Earth is flat. This belief likely comes from a misunderstanding regarding the meaning of the numbers that come after the word nylon.
To the uninitiated, it may seem reasonable that a material called nylon 6/6 (or 66), when it degrades, loses one of the 6s to become nylon 6. Not true. Nylon 6 and nylon 6/6, despite their physical and chemical similarities, are different polymers made in very different ways.
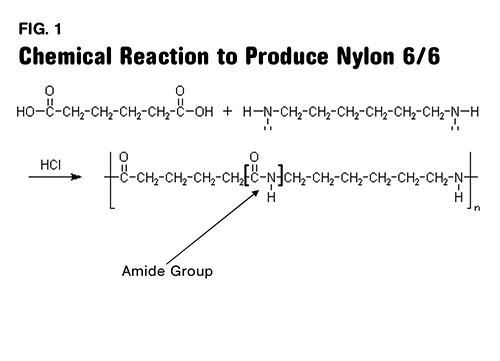
Nylon 6/6, and all other nylon polymers with a nomenclature that involves two numbers, such as nylon 6/12 or nylon 4/6, is produced by reacting two different chemicals to form a precursor. This material is then polymerized to form the final material. The numbers after the word nylon signify the number of carbon atoms that are present in each of the original reactants. Nylon 6/6 begins as a diamine known as 1,6-hexane diamine (or hexamethylenediamine) and a diacid known as hexanedioic acid (or adipic acid).
The diamine contains six carbons and the diacid contains six carbons, as suggested by the “hexa-” root in both names, so when the polymer is formed there are two distinct segments in each repeating unit of the polymer chain that are six carbons in length.
Figure 1 shows the reaction and if you have the patience, you can count out the six carbons in each reactant as well as in the repeating unit of the chain segment.
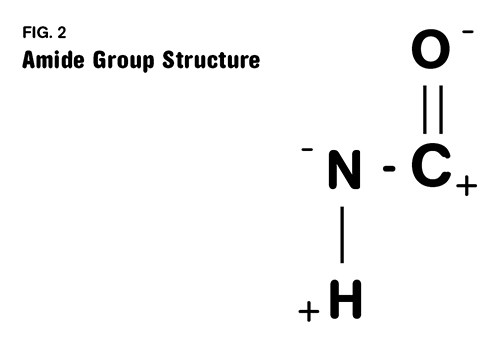
One of these carbons is part of the key chemical group that gives nylon its distinct properties. This group is called an amide group (in most of the world nylons are called polyamides) and it is responsible for the relatively high melting point, strength, and affinity for moisture that nylons exhibit. This group is highlighted in Fig. 1 and shown in more detail in Fig. 2. The first number refers to the diamine and the second one refers to the diacid. So in nylon 4/6 the same diacid is used that is employed in making nylon 6/6, but the diamine only contains four carbons. This decreases the spacing between the amide groups, resulting in a material that is stronger, stiffer, and has a higher melting point than nylon 6/6.
In the case of nylon 6/12, the diamine remains the same but the diacid that is used contains 12 carbons. This increases the spacing between the amide groups, resulting in decreased mechanical properties and melting point. But because the amide group is responsible for the affinity that these polymers have for moisture, nylon 6/12 is more resistant to moisture gain and the associated changes in dimensions and mechanical properties that are experienced with nylon 4/6 and nylon 6/6.
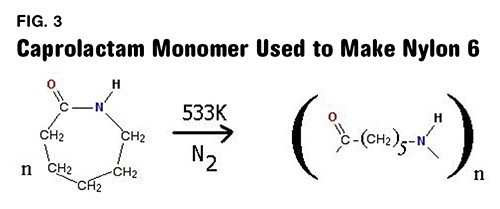
Nylon 6 and other nylon polymers that contain only a single number in the name are made in a completely different manner. In these cases a single chemical is used that has a ring structure and contains both of the chemical groups needed to produce the amide functionality. Figure 3 shows the chemical reaction used to produce nylon 6. The monomer is known formally as aminocaproic acid, but in its ring form it is called caprolactam. When the ring is opened the chemical can react with itself to produce the nylon 6 polymer. Note that this repeating unit also contains six carbons, five that are attached only to hydrogen while the sixth one is part of the amide group.
Even though the average spacing between amide groups is the same in nylon 6 as it is in nylon 6/6, the nylon 6/6 has a melting point approximately 40oC higher and is slightly stronger and stiffer than nylon 6 because of details in the molecular spacing that are beyond the scope of this article. But the bottom line is that nylon 6 and nylon 6/6 are completely different materials made by completely different routes. While they can be combined to produce a wide range of properties, they cannot be converted into one another. When nylon 6/6 degrades it produces by-products that ultimately resemble the chemicals from which it was produced, not nylon 6.
Right now everyone is talking about nylon 12. The shortage in the world market was caused by an explosion at a plant that makes a chemical known as cyclododecatriene (CDT). Through a series of chemical reactions, CDT, a 12-carbon ring structure, becomes a chemical known as laurolactam, a 12-carbon ring with the same amide functionality built into it that is contained in caprolactam.
But when nylon 12 is made, the amide groups are twice as far apart as they are in nylon 6. This results in a material that has a melting point 50-60°C lower than nylon 6. But more importantly this material is much more flexible so that it can be made into hose, tubing, and even balloon catheters. By the same mistaken logic as was mentioned above, where nylon 6 supposedly forms when nylon 6/6 degrades, we should be able to solve the nylon 12 shortage by simply degrading some nylon 6/12. Better yet, by simple math it should be obvious that nylon 6/6 can be converted into nylon 12. Easy, right?
Obviously not. The automotive industry summit meeting in Detroit in mid-April to discuss the nylon 12 shortage was not convened because this is going to be simple. And while automotive is getting most of the attention in the press, it will be medical device suppliers that get to stand first in line when the limited supply is doled out. If other nylons do successfully stand in for nylon 12, (nylon 11 makes the most sense but is also in short supply) it will be due to a combination of skillful manipulations of chemistry coupled with tough decisions about design, processing, and even the consequences of using additives to optimize performance. Chemistry is seldom as simple as it looks. Polymer chemistry takes the complexity up a notch. Nylon chemistry is about much more than doing the math.
Related Content
Scaling Up Sustainable Solutions for Fiber Reinforced Composite Materials
Oak Ridge National Laboratory's Sustainable Manufacturing Technologies Group helps industrial partners tackle the sustainability challenges presented by fiber-reinforced composite materials.
Read MoreThe Fantasy and Reality of Raw Material Shelf Life: Part 1
Is a two-year-old hygroscopic resin kept in its original packaging still useful? Let’s try to answer that question and clear up some misconceptions.
Read MoreTracing the History of Polymeric Materials: Aliphatic Polyketone
Aliphatic polyketone is a material that gets little attention but is similar in chemistry to nylons, polyesters and acetals.
Read MoreLanxess and DSM Engineering Materials Venture Launched as ‘Envalior’
This new global engineering materials contender combines Lanxess’ high-performance materials business with DSM’s engineering materials business.
Read MoreRead Next
People 4.0 – How to Get Buy-In from Your Staff for Industry 4.0 Systems
Implementing a production monitoring system as the foundation of a ‘smart factory’ is about integrating people with new technology as much as it is about integrating machines and computers. Here are tips from a company that has gone through the process.
Read MoreWhy (and What) You Need to Dry
Other than polyolefins, almost every other polymer exhibits some level of polarity and therefore can absorb a certain amount of moisture from the atmosphere. Here’s a look at some of these materials, and what needs to be done to dry them.
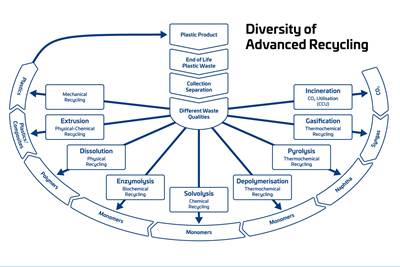
Read MoreAdvanced Recycling: Beyond Pyrolysis
Consumer-product brand owners increasingly see advanced chemical recycling as a necessary complement to mechanical recycling if they are to meet ambitious goals for a circular economy in the next decade. Dozens of technology providers are developing new technologies to overcome the limitations of existing pyrolysis methods and to commercialize various alternative approaches to chemical recycling of plastics.
Read More.jpg;width=70;height=70;mode=crop)