The Need for Generalists, Part 2
Problem-solving requires a team that combines people having academic credentials with others who have hands-on experience.
There is a large gulf in the plastics industry between those professionals who are classically trained in an academic environment and those who gain their experience in industry. Both arenas have value, but they are very different, and the manner in which people learn in these two settings is also very different.
It is the rare individual who has the opportunity to gain a significant amount of experience in both settings. Those who do stand out, like the late, great Myer Ezrin, Stephen Driscoll at Univ. of Mass.-Lowell, and design consultant Glenn Beall, are all reminders of how versatile a professional in the world of plastics can be. But these examples are few and far between, and their numbers are dwindling.
No one can be an expert in everything. Even in the relatively small piece of the known world that polymers represent, there is an enormous amount to grasp. Complicating this picture is the fact that many people active in the plastics industry had no intention of going into plastics and did not train for it. If you poll a representative cross-section of people at any trade show or technical conference, you will find people with degrees in mechanical engineering, electrical engineering, chemistry (often not polymer chemistry), material science (usually all about metals), computer science, and economics, to name just a few. If any of these people have an advanced degree, it is just as likely to be an MBA as anything else.
If you put enough of these people of varied backgrounds in the same room with the right corporate climate, you have an outstanding problem-solving team. Unfortunately, it takes an inordinate amount of managerial talent and focus to keep such a group focused and functioning. Instead, things tend to devolve into the silos that we all say we abhor, and individually we tend to proceed down career paths that become increasing narrow and out of touch with colleagues who do not look, think, and act just like us.
The result of all this—when it comes to the analytical-services companies that are often responsible for identifying the causes of a problem—is that despite all the impressive equipment, the years of formal education, and the advanced degrees, we often come up short. Take a common problem that is responsible for many product failures—an excessive decline in the average molecular weight of a polymer. Molecular weight is the fundamental property on which polymer performance is based. Unless the molecules are of a certain minimum size. It is not possible to achieve the chain entanglement that is the source of the unusual set of properties that polymers offer. Once this minimum molecular size has been established, increases in molecular weight provide further improvements in performance.
In almost every polymer family there are commercial products that span a range of average molecular weights. While the actual molecular weights are not often talked about, measurements such as melt flow rate and intrinsic viscosity provide a relative assessment of average molecular weight. Polycarbonates, for example, may have melt flow rates as low as 1 g/10 min (high molecular weight) to as high as 80 g/10 min (low molecular weight). The high-molecular-weight materials are used to make products where long-term durability is needed, while the low-molecular-weight materials go into intricate shapes with low mechanical demand, such as optical storage media.
Each commercial grade of material is made to a certain target molecular weight. If we assume that the person selecting the material has made a good choice, a part properly molded from that material will perform as intended. However, improper processing can cause excessive reductions in the average molecular weight of the polymer. This can result in a loss in ductility as well as reductions in long-term performance characteristics such as fatigue resistance and environmental stress-crack resistance. Therefore, molecular-weight testing should be part of any product-failure investigation. But this step is often bypassed in favor of more complex testing that does not address this particularly important characteristic. If it is performed, the next hurdle is getting someone to interpret the results so that a decision can be made on whether the change in molecular weight is part of the problem.
A common complaint on the part of clients who receive lab results is that there is little or no explanation regarding the significance of the measurements that are taken and reported. For example, the analyst may report that the melt flow rate of the raw material is 15 g/10 min and the MFR of the molded part is 30 g/10 min, without commenting on whether or not this is an appropriate outcome. Molecular weight is covered extensively in the textbooks on polymers. There are discussions on primary and secondary methods of measurement and the chemical reactions by which appropriate molecular weights are achieved.
But there is virtually no guidance on what is needed on a practical level to ensure proper functioning of a product. That information has come from a vast amount of practical experience gained through empirical testing, and that work has not been incorporated into the formal texts that are used in the academic arena. So a student pursuing an advanced degree in polymer chemistry can read thousands of words about the topic of molecular weight and study all the appropriate equations without ever encountering a discussion about the quantitative relationship between average molecular weight and product performance.
When addressing a large group of technical people who work for a major resin supplier, I put the question to them of what represents the maximum allowable decline in average molecular weight from raw material to fabricated part. They answered immediately and almost unanimously that the maximum allowable reduction is 10%. How do they know this with such certainty? Because they have done the work. They have correlated physical properties to average molecular weight and have observed that product performance begins to decline when this point is reached.
And yet I have seen many test results that report molecular-weight reductions of 20% to as high as 50% and there is not one word regarding the implication of this finding regarding the performance of the part. There is so little formal treatment of this topic that a colleague of mine was involved in a legal case some years ago where their tests showed a 50% reduction in the molecular weight of the polymer and brought that to trial as evidence of a major problem with product performance. When the “expert” for the opposing side testified that this level of change in molecular weight was not problematic, an extensive literature search found nothing on which to base a rebuttal of that claim. As a result, the case dragged on for weeks and cost all involved a great deal of money while the smoking gun sat unnoticed.
It isn’t that the knowledge is not out there relating molecular weight to performance. There are hundreds if not thousands of professionals in the industry who can connect the dots between a large reduction in average molecular weight and a product failure. But they are not usually the ones doing the lab testing and writing the reports. Someone with both an academic understanding and the practical experience, or a team that contains both the highly trained analytical chemist and the field person with practical know-how, is needed to draw the appropriate attention to the problem.
Next month we will look at some other instances of missed opportunities brought about by an overly narrow focus (Read Part 1 Here).
ABOUT THE AUTHOR Mike Sepe is an independent, global materials and processing consultant whose company, Michael P. Sepe, LLC, is based in Sedona, Ariz. He has more than 40 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 • mike@thematerialanalyst.com.
Related Content
How to Select the Right Tool Steel for Mold Cavities
With cavity steel or alloy selection there are many variables that can dictate the best option.
Read MoreThe Effects of Temperature
The polymers we work with follow the same principles as the body: the hotter the environment becomes, the less performance we can expect.
Read MoreWhy (and What) You Need to Dry
Other than polyolefins, almost every other polymer exhibits some level of polarity and therefore can absorb a certain amount of moisture from the atmosphere. Here’s a look at some of these materials, and what needs to be done to dry them.
Read MoreTunnel Gates for Mold Designers, Part 1
Of all the gate types, tunnel gates are the most misunderstood. Here’s what you need to know to choose the best design for your application.
Read MoreRead Next
The Need for Generalists: Part 1
Even companies with seemingly unlimited resources can have problems identifying root causes of problems. Maybe they have too many specialists and not enough generalists.
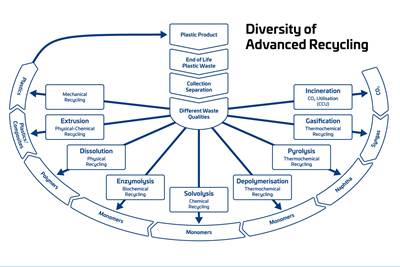
Read MoreAdvanced Recycling: Beyond Pyrolysis
Consumer-product brand owners increasingly see advanced chemical recycling as a necessary complement to mechanical recycling if they are to meet ambitious goals for a circular economy in the next decade. Dozens of technology providers are developing new technologies to overcome the limitations of existing pyrolysis methods and to commercialize various alternative approaches to chemical recycling of plastics.
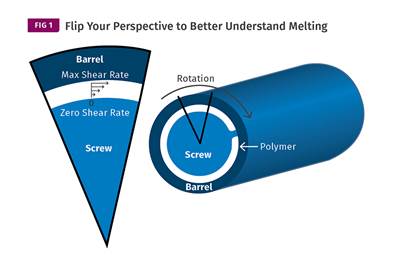
Read MoreUnderstanding Melting in Single-Screw Extruders
You can better visualize the melting process by “flipping” the observation point so that the barrel appears to be turning clockwise around a stationary screw.
Read More.jpg;width=70;height=70;mode=crop)