Automation Eliminates Contamination
Many molders use robots to boost output and repeatability, but SSI also uses them to prevent contamination of critical diagnostic products.
“We use automation a little bit differently than most injection molders,” says Anthony McCracken, director of operations for Scientific Specialties, Inc. (SSI), Lodi, Calif. “Because our customers are testing for DNA, it is absolutely critical that there is no contamination on the products we manufacture. Where there are people, there is the potential for DNA contamination. Sure, robots don’t get tired and they don’t need to take breaks, so they do contribute to production efficiency. But the really critical thing for SSI is to put a barrier between the molding machines and the operators, and our robots allow us to do that very effectively.”
SSI has worked with Sepro America, Warrendale, Pa., as its sole automation supplier, using Sepro’s servo-driven robots and downstream systems to eliminate the potential for human contamination. Running pipette-tip molds with up to 32 cavities on cycles as short as 8 sec pushes SSI’s production north of several million individual products per day. Running at those rates makes scrap prohibitively expensive.
SSI has 12 Sepro robots, including nine that are installed on machines without downstream automation or with automation developed in-house. SSI operates 46 Arburg and three Negri Bossi injection machines, ranging in size from 50 to 220 tons.
SSI has three automation cells making pipette tips. It first called on Sepro to supply three-axis S5-15 robots. Then, almost three years ago, Sepro designed the first of three post-mold automation systems to work in conjunction with the three-axis systems SSI already had in place.
In operation, the robot’s end-of-arm tooling (EOAT) removes pipette tips from the mold. Because the tips are gripped at the narrow end, opposite the base, but need to go into their racks in the opposite orientation, the EOAT hands the tips off to a second fixture outside the molding machine for reorientation.
This fixture tilts 90° so it can place the tips point-down into a third fixture that shuttles them away from the molding machine and positions them above plastic drop tubes that guide them into their racks in the correct orientation. Each rack holds 96 tips, with a servo drive indexing the racks to receive three sequential drops of 32 tips. Along the way, a camera is used to automatically inspect each pipette tip, automatically removing any that fail.
All the automation cells were assembled at Sepro’s facility outside Pittsburgh and tested prior shipment to SSI in California. McCracken says he has another Sepro cell on order for delivery this month. This newest cell will include some additional features to facilitate quick mold change, since SSI produces pipette tips in several different sizes and wants to reduce changeover times from several hours to “just a few hours or less,” says McCracken.
Founded in 1990 by K.R. Hovatter, and his wife, Robbie, SSI competes in a global and highly competitive market with 100% U.S.-based production, thanks in large part to automation. “Time after time, we have seen companies try to take advantage of low labor cost in other countries, including China,” McCracken says. “But the issue of quality trumps labor costs in this industry. That’s why we’ve opted instead to put money into automation that allows us to be competitive while maintaining a very high quality standard and remaining a 100% domestic manufacturer.
Related Content
Catheter Specialist Finds Sweet Spot Serving Small, Medium-Sized Concerns
Medical-component specialist LightningCath has carved a niche meeting the needs of small to medium-sized entrepreneurs with complex catheter designs … quickly.
Read MoreUse Cavity Pressure Measurement to Simplify GMP-Compliant Medical Molding
Cavity-pressure monitoring describes precisely what’s taking place inside the mold, providing a transparent view of the conditions under which a part is created and ensuring conformance with GMP and ISO 13485 in medical injection molding.
Read MoreSeaway Plastics Acquired
Private equity firm ICG purchased the injection molder and its three facilities located in Florida and California.
Read MoreMedical Manufacturer Innovates with Additive Manufacturing and Extrusion Technology Hubs
Spectrum Plastics Group offers customers two technology hubs — one for extrusion, the other for additive manufacturing — to help bring ground-breaking products to market faster.
Read MoreRead Next
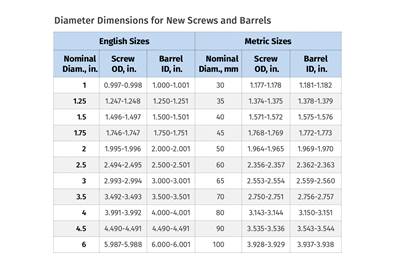
Troubleshooting Screw and Barrel Wear in Extrusion
Extruder screws and barrels will wear over time. If you are seeing a reduction in specific rate and higher discharge temperatures, wear is the likely culprit.
Read MorePeople 4.0 – How to Get Buy-In from Your Staff for Industry 4.0 Systems
Implementing a production monitoring system as the foundation of a ‘smart factory’ is about integrating people with new technology as much as it is about integrating machines and computers. Here are tips from a company that has gone through the process.
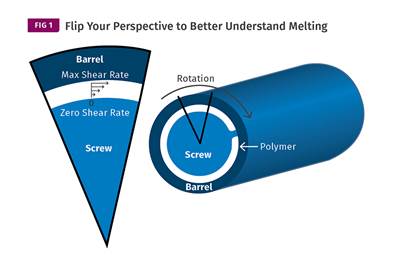
Read MoreUnderstanding Melting in Single-Screw Extruders
You can better visualize the melting process by “flipping” the observation point so that the barrel appears to be turning clockwise around a stationary screw.
Read More










.png;maxWidth=300;quality=90)