Compression Blow Forming: Promising New Process
‘Game-changing’ technology is overcoming initial growing pains with a new generation of machines.
Willingness to invest in brand-new and potentially “game-changing” process technologies is one aspect of the strong emphasis on R&D at Amcor Rigid Plastics, Ann
Arbor, Mich. As reported in last month’s profile of the company, one of the firm’s pioneering projects is Compression Blow Forming, which Amcor was the first to put into commercial production in 2012. A recent conversation with Tod Eberle, v.p. of Quality Systems, Engineering Standards, and Advanced New Platform Technologies, provided an update on where this technology stands today and on progress toward realizing its promise of superior quality, productivity, and sustainability.
PHARMA BOTTLES BENEFIT
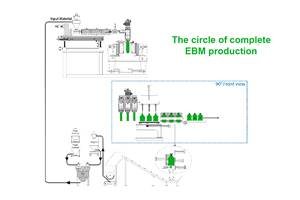
Compression Blow Forming (CBF) technology evolved from continuous compression molding of caps and closures by Sacmi of Italy (see July’12 Close Up). CBF involves continuous extrusion of a “hockey puck,” which is compression molded into a preform
and then stretch-blown in a system of interlocking wheels. Amcor has three 12-cavity CBF machines in Youngsville, N.C., making stock and custom HDPE pharmaceutical bottles for two large producers of generics and supplements.
Eberle has no doubt that CBF will prove to be a game changer. It has demonstrated energy savings of 30-35% compared with the injection-blow molding (IBM) process normally used for pharma bottles. “Customers like the sustainability benefit,” Eberle notes. CBF also offers higher productivity—up to 6000 bottles/hr—with higher rates on the horizon. “Dramatically” tighter weight control is another benefit. For a typical IBM
bottle-weight specification of ± 0.7-0.8 g, CBF achieves a Cpk of 10, which is “unheard of” in IBM, says Eberle. He also likes the extremely low scrap generation on startup and shutdown—“the lowest of any polyolefin process I’ve seen.”
Amcor set specific goals for the process: performance to nameplate capacity of 85% or better and manufacturing efficiency (uptime) greater than 90%. Both have been realized, he says, with some qualifications.
ADDRESSING WEAR ISSUES
As with any brand-new process, not to mention one as sophisticated and complex as this, there have been growing pains. “The primary long-term issue has been premature wear of the aluminum blow molds,” Eberle acknowledges. “We’re cutting IBM cycle times by around half, so we need fast-cooling aluminum molds. But aluminum tends to be soft, which leads to parting-line wear and visual defects in bottles. Unfortunately,
pharma customers are used to perfect parting lines from IBM.”
The second major concern has been wear on the rotary union that distributes air, hydraulic fluid, and cooling medium at two different temperatures. Eberle is quick to note that “Sacmi has been world-class in working with us to address these issues.” He is pleased that Sacmi supplemented its spare parts facility in Des Moines, Iowa, last year with a sales office in Atlanta, giving it added presence in North America.
Eberle says there has been steady progress in improving the mold engineering. The next-generation machine, now being tested by Sacmi, uses a clamshell closing arrangement in place of the parallel closing used to date. This is expected to be gentler to the molds. “We’re targeting a three-year refurbishment life for blow molds, but we’ll need more time to prove it.”
The new machines have 16 to 20 cavities and also provide 8-15% shorter dry-cycle time, promising significant productivity enhancement. Like the first-generation systems, these are servo-electric/hydraulic hybrids, but Sacmi is well along in developing an all-electric version that may debut in 2017.
The upshot from three years of production experience is that CBF makes good sense for dedicated production of high-volume products, Eberle concludes. Changing over compression and blow tooling, and perhaps the extrusion nozzle and downstream handling, is more complex and time-consuming than for IBM. “We’ll reserve IBM for frequent-changeover, shorter runs.”
Related Content
As Currier Grows in Medical Consumables, Blow Molding Is Its ‘Foot in the Door’
Currier Plastics has added substantial capacity recently in both injection and blow molding for medical/pharmaceutical products, including several machines to occupy a new, large clean room.
Read MoreUnderstanding the ‘Science’ of Color
And as with all sciences, there are fundamentals that must be considered to do color right. Here’s a helpful start.
Read MoreHow Inline Vision Inspection Can Minimize Scrap in Molding
Once viewed by injection and blow molders as a necessary evil, machine vision technology today can continuously monitor and improve production while reducing costs.
Read MoreGet Color Changes Right In Extrusion Blow Molding
Follow these best practices to minimize loss of time, material and labor during color changes in molding containers from bottles to jerrycans. The authors explore what this means for each step of the process, from raw-material infeed to handling and reprocessing tails and trim.
Read MoreRead Next
Compression Blow Forming Process in Commercial Production
Expectations of higher productivity, improved quality, and energy savings for a novel blow molding process are now being realized in commercial production at Amcor Rigid Plastics in Youngsville, N.C.
Read MoreDeveloping Tomorrow’s Containers: Inside Amcor’s R&D Center
How award-winning package design, full-service laboratory facilities, and game-changing process development support a world-class injection and blow molder.
Read MoreCompression Blow Forming Process in Commercial Production
Expectations of higher productivity, improved quality, and energy savings for a novel blow molding process are now being realized in commercial production at Amcor Rigid Plastics in Youngsville, N.C.
Read More.png;maxWidth=970;quality=90)