Mack Moves Into Medical Disposables With New Cleanroom
At a customer’s request and after a $2 million investment in people and technology, custom molder and contract manufacturer Mack Molding, Arlington, Vt., is making its first foray into high‐volume, single‐use medical components.
In April, the company’s MackMedical division will begin validation runs of a Class III medical device manufactured in a new Class 100,000 (ISO Class 8) 3500‐sq‐ft cleanroom.
That molding and assembly cleanroom is the third at Mack’s headquarters plant in Vermont, and will it house four all-electric injection molding machines from The Japan Steel Works (JSW), bringing the company’s cleanroom press total to 11.
In a novel setup, an additional, automated 500‐ton Engel press outside the clean room will feed a larger component needed for the device into the controlled space from a soft‐walled cleanroom. In addition, servo‐controlled radio frequency and ultrasonic welding systems will be installed, as well as automated particulate, temperature and humidity monitoring.
Molding and assembly operations will include overmolding of an extruded three-lumen tubing, with the presses vacuum‐fed resin by a modular bank of dryers located just outside the cleanroom.
Jeff Somple, president of Mack Molding, and Kevin Bradley, business unit director of MackMedical told Plastics Technology that the 500-ton press would have changed the ceiling height requirements of the cleanroom and required the addition of an overhead crane. To meet the job requirements without significantly altering the cleanroom space, Mack devised the more flexible solution of the soft‐walled space.
Once fully built out and given customer demand, Mack expects the newest cleanroom to feature a total of eight injection molding machines, with six presses inside the clean room, adding two presses there, as well as two machines outside, with an additional larger machine deployed.
The four new all-electric JSWs include two 44‐ton vertical machines—the first verticals at that site—and 60‐ and 199‐ton horizontal presses. The machines are from JSW’s ‘advanced’ series and feature a 62 micro second servo control circuit for high‐speed performance, increased precision and reliable quality, according to Mack. The first machine installations bring Mack’s cleanroom press total to 11.
Mack other two other Class 100,000 cleanrooms include a molding cleanroom with six all-electric presses and an assembly cleanroom for non‐sterile packaging of medical disposables, light sonic weld assembly, and temperature‐ and humidity‐ controlled functional testing. There is also a 24‐hour whiteroom operation with four hydraulic presses dedicated to small part medical molding.
Anticipating More Business
Somple and Bradley believe the Spring completion and validation of the new cleanroom space, including large-part molding capacity, will create many new opportunities for Mack in the medical sector. “Once people see the complexity of the additional space,” Bradley said, “it really will open up the floodgates.”
Somple reiterated the growth potential and described the conflicting interests between molders and potential customers surrounding cleanrooms as a classic “Catch 22.”
“Customers don't want to be the first ones to break in your cleanroom,” Somple noted, “but you have to have a cleanroom to attract the customers.” He believes that going forward the room will provide opportunities for jobs involving hundreds of thousands of parts from four and eight-cavity tools, versus millions from an application like a 64-cavity closure.
Mack first moved into the medical market back in 2000 as it exited the computer and business equipment space, watching the segment’s revenue share climb from nothing to 40% of its business.
Investing in Human Capital
Mack also augmented its medical device engineering expertise with new hires and realigned staffing to support the growing segment. David Clatworthy, formerly of AngioDynamics Inc., and Timothy Hutchings, formerly of Covidien/Tyco Healthcare, have been hired, bringing more than 50 years of combined engineering and manufacturing experience to Mack. Clatworthy will have manufacturing engineering responsibilities for both single use and reusable orthopedic product lines at Mack, while Hutchings will have manufacturing engineering responsibilities for medical device disposables.
Both will report to Scott Hodges, who has recently been promoted to manufacturing engineering manager. A 20-year Mack veteran with experience in both quality and manufacturing engineering, Hodges most recently was responsible for all engineering, operations, production planning and compliance for the manufacture of several FDA Class III medical devices.
Hodges’ promotion is part of a recent manufacturing reorganization designed to separate engineering from production at the headquarters plant. In the past, Mack said engineers were taking on production and scheduling responsibilities, whereas going forward they will focus on cost reductions, continuing improvement efforts, and new program launches.
Related Content
Artificial Intelligence Enables Smarter Sourcing
Westfall Technik has adopted Arkestro’s predictive procurement software to wring savings and more reliable deliveries from a historically challenging supply chain.
Read MoreNew Cap for Child-Resistant Pill Bottles Is Senior-Friendly & Saves Resin
Exclusive lightweight cap design can be removed simply pushing down on the center. No gripping or twisting needed.
Read MoreWisconsin Firms Unite in Battle Against Covid
Teel Plastics opened new plant in record time, partnering with AEC & Aqua Poly Equipment Co. to expand production of swab sticks to fight pandemic.
Read MoreKrones Acquires Netstal
Krones adds PET preform injection molding to its bottle blowing and filling capabilities, as well as cap molding and expansion into medical, food and other markets.
Read MoreRead Next
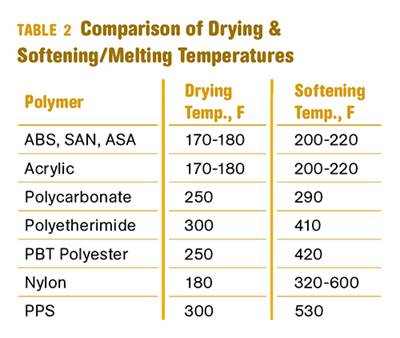
Why (and What) You Need to Dry
Other than polyolefins, almost every other polymer exhibits some level of polarity and therefore can absorb a certain amount of moisture from the atmosphere. Here’s a look at some of these materials, and what needs to be done to dry them.
Read MoreLead the Conversation, Change the Conversation
Coverage of single-use plastics can be both misleading and demoralizing. Here are 10 tips for changing the perception of the plastics industry at your company and in your community.
Read More












.png;maxWidth=300;quality=90)