Prototype to Production: NPI/Medical Is Quick-Turn Artist
New name, same focus: From 1 part to millions, a new-product development manufacturer for over 45 years.
The name says it all: New Product Introduction (NPI), with a focus on Medical. Or maybe not quite all: NPI/Medical is really two businesses—production molding for medical markets and Quick-Turn Manufacturing (QTM), or low-volume molding from prototype to pre-production “bridge” stage.
What the two businesses have in common is a focus on speed, service, and technical sophistication. For speed, NPI/Medical relies on a substantial in-house tooling department, which can produce single-cavity, tight-tolerance steel prototype molds in two to four weeks. At NPI/Medical, the word “prototype” could give the wrong impression—these tools mold production-quality parts within a prototype time frame.
Service encompasses NPI/Medical’s eagerness to support low-volume production, often for startup companies, and its wide range of capabilities to support varied customer demands. As president David Kelly puts it, “When a customer asks whether we have a certain capability, we want to say yes.”
• Do you have in-house product and tool engineering? Yes.
• In-house tooling capabilities? Yes.
• Offer material-selection advice? Yes.
• Handle difficult engineering resins? Yes.
• Liquid silicone rubber? Yes.
• Insert molding? Yes.
• Multimaterial overmolding? Yes.
• Ship products globally? Yes.
• Provide decorating, welding, assembly, and packaging? Yes.
• Clean-room molding and assembly? Yes.
• In-house metrology services? Yes.
• Vendor-managed inventory? Yes!
As for technical sophistication, Kelly says, “If a customer needs a PC/ABS cover, they don’t even think of us. We’re the company that can do the tough stuff. Our niche is tight tolerances and engineering materials—PEEK, PPS, Ultem polyetherimide (PEI), Radel polyphenylsulfone, liquid-crystal polymer (LCP). It’s one of the reasons people come to us.”
REFOCUSED ON MEDICAL
NPI/Medical has occupied a 66,000 ft² plant in Ansonia, Conn., since 1999. The building, sitting on a 13-acre site, could easily be expanded to 85,000 ft², according to project manager William Ball.
The plant employs 95 and houses 46 injection machines from 28 to 300 tons. The firm’s revenues are split 70-30 between production molding and QTM development work. Kelly projects double-digit growth for the next five years and has budgeted for two more presses this year. Says Ball, “This existing plant can support growth to $35 million in annual business.”
Certified to ISO 13485:2003 and ISO 9001:2008 quality standards, NPI/Medical does work for both small startups and large medical OEMs. Kelly notes that a wave of consolidation among medical OEMs is putting additional demands on molders serving them.
“When one OEM buys another, every vendor has to defend its position in the supply chain. And you must have the flexibility to adopt the new protocols of the acquiring company.”
Despite this industry consolidation, NPI/Medical has not lost any business, and has even picked up some from “molders that are more commoditized than us,” Kelly says, “or ones that do other things besides medical, like automotive. It helps to specialize.”
Kelly, who has spent 30 years in plastics, the last 15 of them at NPI/Medical and its predecessor company, points out that the firm wasn’t always so specialized—at least not on medical products. The NPI/Medical name dates only from September 2013, when it was spun off from Minneapolis-based Spectrum Plastics Group. According to Kelly, “Changing the name was a very good thing for us, because Spectrum had been associated with a very different kind of business.”
Back in the 1970s, Spectrum’s core business was electronics, specializing in reel-to-reel molding of connectors, microsensors, open-cavity packages, and LED lenses onto stainless steel and plated copper strips (a technique developed by Spectrum).
“We made millions per week,” Kelly recalls, “then it all went overseas in 2009. So we re-focused on medical.”
In particular, NPI/Medical concentrates on surgical and diagnostic devices, as well as life-sciences products—DNA sequencers, filters, etc. “We’re not a commodity medical molder—no 64-cavity syringes here,” says Kelly. “We use up to eight cavities in production, and our machines go up to 300 tons.” Prototyping at NPI/Medical is generally in single-cavity tools, except for family molds.
QTM AND P2P
NPI/Medical prides itself on its appetite for short runs. “If you need hundreds of parts, we’ll do it,” says Kelly. “We have medical orders for as few as 400 parts a year. We have a huge job coming up to mold 15 different parts and assemble them for just 250 finished assemblies a year!”
Kelly continues, “We support startups. A lot of big companies won’t do that. Our bigger competitors don’t want to deal with low volumes. We even offer medical validation on low-volume development work—say, 1500 parts a year. A lot of companies won’t even consider that.”
Low- to mid-volume molding is integral to the firm’s QTM business, which it defines as “1 to 500,000 parts.” The essence of this business segment is helping clients get new product ideas off the ground fast. “When a medical or other OEM has an idea, we want them to come to us. We can help them get there. When they are in design and development chaos, that’s where we step in. They can count on us.”
NPI/Medical offers QTM clients its DynaClass Tooling System, consisting of four levels of tooling from DynaClass 1 for “form, fit & function” prototypes (up to 5000 parts), deliverable in one to three weeks; to DynaClass 2 molds for tight-tolerance, advanced prototypes (up to 50,000 parts), suitable for validation, which take two to four weeks; and DynaClass 3 and 4 low-volume and pre-production bridge tooling for up to 100,000 and 500,000 parts, respectively, with lead times from three to eight weeks. (These tooling classes correspond to SPI Classes 105 to 102.) Standard tolerances for all DynaClass tools and parts are ±0.005 in.
Seven JSW presses from 40 to 85 tons are dedicated to QTM business, though NPI/Medical will use its larger production equipment when necessary. Clean-room molding options are available for all but the lowest DynaClass level.
QTM can be considered the development arm of the firm’s New Product Introduction (NPI) business, also referred to as Prototype to Production (P2P). “We have customers that do continuous new-product introductions. We want to get in early to help them realize their concepts,” says Kelly. “And we want them to stay with us for long-run production.
“But we also do development work for customers we know we won’t do production for, such as those that have in-house molding capability or already have a production molder signed up. In some cases, we do QTM development for high-volume, precision industrial applications that aren’t suitable for production here. Like batteries, for instance. I don’t want my production machines tied up with anything but medical business.”
Kelly notes that there are good reasons for a QTM client to stick with NPI/Medical for full production. “If you change vendors after the development stage, you lose everything that was learned to date about how to process the part. You’re starting out afresh.”
Kelly explains another synergy between QTM and production molding. “QTM supports us through our customers’ long development cycle before we get the production order. It takes only a few weeks to build a prototype tool, but the time frame from concept to prototype to testing, design changes, validation, and then production can be 24 to 36 months.”
MANY TALENTS UNDER ONE ROOF
The plant’s operations are divided into four “cells” in separate rooms. Narrow corridors between the cells allow equipment to be moved in and out without disturbing production.
The largest press is a 300-ton Milacron Vista Hydraulic machine. Most of the other larger presses—85 and 110 tons—are all-electric models, including nine Roboshot 110-tonners from Milacron/Fanuc . The rest are mainly Arburg’s and one Nissei press.
A Novatec central drying system was recently reconfigured after the separation from Spectrum Plastics. Transitioning out of most of the reel-to-reel business and putting more emphasis on QTM development brought a need to dry more materials in smaller amounts. Mounted on a mezzanine, the system has 36 drying hoppers divided among three hopper banks, each fed by a wheel-type desiccant dryer. Numbered flexible tubes from each hopper can be connected to any of 43 numbered tubes leading to molding workcells.
Production monitoring, scheduling, and lot control (essential for medical jobs) are managed by a central ERP system from IQMS, which has been in place for eight years. Kelly is looking for systems that give him more data on his operations. For future machine purchases, he’s interested in controls that capture more process data that he can access from his office desktop. He also likes the fact that one of his newer machines, a 110-ton Wittmann Battenfeld EcoPower hybrid press, has capability for remote diagnostics via the Internet.
NPI/Medical has three certified clean rooms—one permanent Class 8 (Class 100,000) clean room equipped with six injection machines of 40 and 110 tons, and two Class 7 (Class 10,000) clean rooms for assembly, testing, and packaging. One of those is a permanent structure of 1150 ft², the other a “soft-wall” enclosure of 600 ft² for prototype and short-run work. In addition, the firm can put a hood over an individual press or cell to create a localized clean area.
NPI/Medical has ample experience in special molding techniques, like insert molding and multimaterial overmolding. In both cases, it uses mostly hand loading of the tools, due to the prevalence of short to medium runs.
The plant has four 55-ton Arburg presses for LSR (convertible for thermoplastics), one of them dedicated to low-volume and prototype work. “We do LSR in low and high volumes,” says Kelly. “It’s a core competency of ours. I’m not talking about O-rings or baby nipples. We do complex geometries and overmolding onto metal or thermoplastics.”
Kelly credits much of NPI/Medical’s expertise in LSR to Tim Erwin, senior technical advisor, who joined the firm about 10 years ago from Pitney-Bowes, where he had worked for 27 years and Kelly for 15. “Tim is one of the industry’s top technical guys in LSR,” states Kelly. “He designed a cold-deck mold frame for LSR to accept interchangeable core and cavity sets. We can build a single core and cavity in four to five weeks for no more than $8000 to $10,000.”
Erwin notes that NPI/Medical’s experience in LSR includes ability to process special materials. A case in point is a liquid fluorosilicone used in DNA sequencers because of its hot-oil resistance. “We’re the only molder of this particular material today,” says Erwin. “It has a viscosity of over 1 million centistokes, versus 600,000 typical for LSR, and it’s not shear-thinning like normal LSR. It’s no picnic.” Even molding standard LSR, Erwin says, is “80% art, 20% science, while thermoplastic molding today is more like 90% science.”
Secondary operations performed by NPI/Medical include ultrasonic welding and pad printing. One hand-held surgical stapler requires 10 “hits” from a pad printer to place labels all around the handle.
The plant’s newest capability is laser etching, which is very fast—one current job etches a set of three parts in 4 sec—and imparts permanent marks that can’t wear off. NPI/Medical uses the laser technique on specially formulated grades of PC, ABS, and nylon.
Assembly and packaging are other core competencies at NPI/Medical. “When we mold several parts for a product, it’s hard to compete with us for the assembly portion of the job,” says Erwin.
A case in point is the plant’s “kitting room,” designed by Erwin, where five models of DNA sequencers are packaged in ready-to-use kits. “We mold six parts and purchase three more, plus the packaging and label,” explains Ball. “We ship around 6000 a year per model. We audit the sub-vendors and provide controlled lot traceability, thanks to our IQMS ERP system. For each order, we send four kits to the customer for approval and hold the rest of the order until we receive the go-ahead. That can take less than two weeks or up to six weeks for a new job.”
Essential to NPI/Medical’s Quick-Turn capabilities is its toolroom, staffed by eight toolmakers and two apprentices. The equipment range comprises six Bridgeport milling machines, seven grinders, six CNC machining centers (including one that can operate lights-out with a six-axis robot to make up to 32 EDM electrodes) and five EDM systems. The latter can carry up to 82 electrodes, and one is a wire EDM machine, not often seen in small shops. Also relatively uncommon is a $30,000 microwelder—a “very big timesaver,” according to Ball.
The toolroom builds eight to 10 QTM molds a month, satisfying 85-90% of the firm’s development needs. However, NPI/Medical outsources all its production tooling to local moldmakers.
Another key capability is the firm’s metrology lab, equipped with four optical and touch-probe coordinate measuring machines. Samples of all parts come through the lab to be qualified. The lab also inspects tooling components and fixtures.
To accommodate his customers’ obsession with quality, Kelly took the unusual step of moving the process engineers and technicians into the quality department. “That means our quality team is accountable for the process as well as the parts. It means the project manager can go to one person—the quality manager—to address any problems.”
Related Content
Medical Manufacturer Innovates with Additive Manufacturing and Extrusion Technology Hubs
Spectrum Plastics Group offers customers two technology hubs — one for extrusion, the other for additive manufacturing — to help bring ground-breaking products to market faster.
Read MoreRecord Reshoring Rates in 2022
Reshoring and foreign direct investment (FDI) in the third quarter marked their highest ever level, eclipsing the previous record set in the second quarter of 2022.
Read MoreMedical Molder, Moldmaker Embraces Continuous Improvement
True to the adjective in its name, Dynamic Group has been characterized by constant change, activity and progress over its nearly five decades as a medical molder and moldmaker.
Read MoreKrones Acquires Netstal
Krones adds PET preform injection molding to its bottle blowing and filling capabilities, as well as cap molding and expansion into medical, food and other markets.
Read MoreRead Next
Lead the Conversation, Change the Conversation
Coverage of single-use plastics can be both misleading and demoralizing. Here are 10 tips for changing the perception of the plastics industry at your company and in your community.
Read MoreHow Polymer Melts in Single-Screw Extruders
Understanding how polymer melts in a single-screw extruder could help you optimize your screw design to eliminate defect-causing solid polymer fragments.
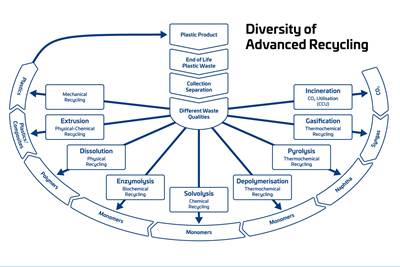
Read MoreAdvanced Recycling: Beyond Pyrolysis
Consumer-product brand owners increasingly see advanced chemical recycling as a necessary complement to mechanical recycling if they are to meet ambitious goals for a circular economy in the next decade. Dozens of technology providers are developing new technologies to overcome the limitations of existing pyrolysis methods and to commercialize various alternative approaches to chemical recycling of plastics.
Read More


























.png;maxWidth=300;quality=90)