Tracing the History of Polymeric Materials: PBT
The slow crystallization of PET polyester made it a poor option for processes like injection molding. This led to the development of more molder-friendly options such as PBT.
While market opportunities for PET polyester in fibers for fabric (the semi-crystalline form) and in beverage bottles (the amorphous form) created substantial growth during the period from the early 1950s to the mid-1970s, the slow crystallization of the polymer made it a poor option for processes like injection molding. Early attempts to create materials for this type of conversion process resulted in poor impact resistance and the need for mold temperatures exceeding 150 C (302 F) to achieve full crystallization.
The first successful attempt to make the material more user-friendly for injection molders came from DuPont in 1978. However, almost a decade earlier, a variation on the PET chemistry was commercialized that circumvented the issues that PET exhibited. This was polybutylene terephthalate (PBT). Celanese was the first company to introduce PBT in 1969, followed closely by GE Plastics in 1972. The initial applications were in electrical and electronic markets, where it was recognized that the material properties as well as dimensions of the molded parts were more stable than those produced in nylon once the materials became equilibrated with atmospheric moisture.
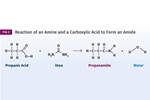
The chemistry of PBT is very similar to that of PET, but there is a subtle difference that brings with it a mixture of advantages and disadvantages. The accompanying figure shows the chemical structure of the repeating unit in PBT. The polymer is produced by essentially the same chemical reaction used to make PET, but with the substitution of butylene glycol for ethylene glycol.
Because butylene glycol is a four-carbon alcohol and ethylene glycol is a two-carbon alcohol, the resulting polymer structure contains a longer segment of the straight carbon chain in each repeating unit. These segments move with greater freedom at a molecular level and thus dilute the strengthening and stiffening effect of the aromatic ring. This enhanced molecular mobility promotes a faster rate of crystallization in PBT. Consequently, PBT is always semi-crystalline in both its filled and unfilled forms.
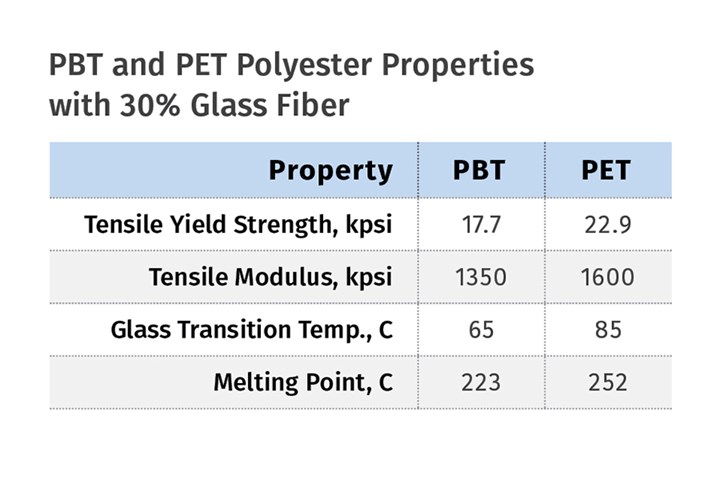
This dilution of the contribution from the aromatic ring comes at a cost that can be observed if the properties of PET and PBT are compared. However, it is important to compare apples to apples. In the unfilled state, PET is typically amorphous and PBT is semi-crystalline. The technology for obtaining a useful level of crystallinity in PET includes the incorporation of fillers, with glass fiber being most commonly used. The accompanying table provides a snapshot of key thermal and mechanical properties for a 30% glass-reinforced grade from each family. These show that once the PET formulation is adjusted to provide for crystallinity, the closer packing of aromatic rings provides a clear advantage in performance.
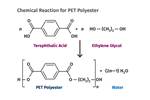
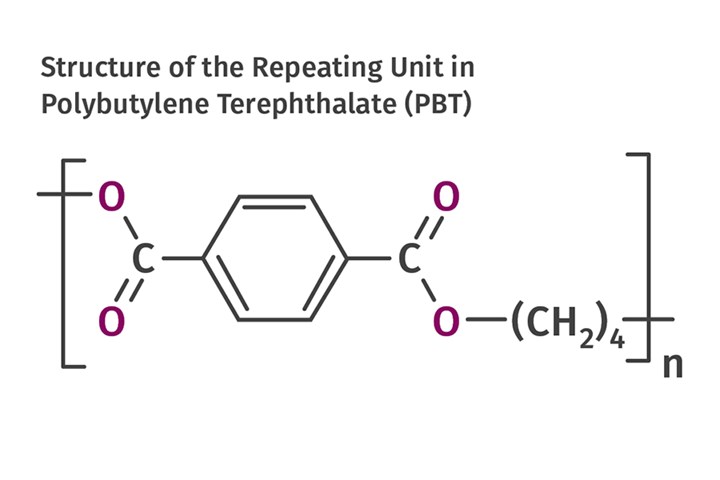 PBT is produced by essentially the same chemical reaction as is used to make PET, but with the substitution of butylene glycol for ethylene glycol. (Source: M. Sepe)
PBT is produced by essentially the same chemical reaction as is used to make PET, but with the substitution of butylene glycol for ethylene glycol. (Source: M. Sepe)This performance advantage drove many end users to move from reinforced PBT to reinforced PET once the slow crystallization problem was solved in 1978. But there were challenges. First, in order to achieve an appropriate level of crystallinity in any semi-crystalline polymer, it is necessary to run the mold temperature above the glass-transition temperature of the polymer. So while PBT can fully crystallize at mold temperatures above 75 C (167 F), PET requires a mold temperature closer to 95 C (203 F). And this is for an optimally nucleated PET. Many material manufacturers that entered the reinforced PET market following DuPont introduced materials that required mold temperatures as high as 120 C (248 F) to obtain full crystallization. This represented a significant barrier for many processors, since it required the use of hot-oil or pressurized-water systems.
Processors familiar with molding PBT often run into problems with polymer degradation when they begin processing PET.
Another notable difference between PBT and PET is their sensitivity to hydrolysis. While crystallizing PET provides a measure of resistance to hydrolysis for use in fibers and fabrics, the material is remarkably sensitive to hydrolytic degradation at melt-processing temperatures. PBT has this same sensitivity, but the rate at which PBT hydrolyzes is significantly slower and processors often get away with molding wet PBT, while molding wet PET almost always results in a disastrous loss of mechanical performance. Processors that are familiar with molding PBT often run into problems with polymer degradation when they begin processing PET. This has led to a lot of myths about the difficulty involved in drying PET when compared with PBT.
But studies have shown that moisture-removal rates in the two polymers at any given set of drying conditions are essentially the same. The difference is that PBT can often tolerate moisture contents that exceed the recommended maximum as long as the melt temperatures are low enough and the barrel residence times are short. PET will not tolerate such conditions. So if a molder is encountering problems with drying PET, it is very likely that they also were having problems drying PBT but may not have noticed. Worse yet, PET reacts so efficiently with excess moisture that it will not exhibit the traditional molding defects associated with wet material. Parts molded from wet PET look as good or better than parts molded from dry PET.
Another interesting distinction between PET and PBT is that for reinforced injection molding grades, PET tends to have a lower melt viscosity and therefore more easily achieves a resin rich finish in the molded parts. This has led to the development of blends of the two polymers to improve the surface finish of the PBT. The Valox 800 series from GE Plastics (now SABIC) was an early example of the use of this blending approach to achieve an optimal surface finish in molded parts, and it is still commercially available.
PBT can often tolerate moisture contents that exceed the recommended maximum as long as the melt temperatures are low enough and the barrel residence times are short.
There is a third type of polyester that fits into this general chemical pattern and is positioned between PBT and PET. It is known commercially as polytrimethylene terephthalate (PTT) but could just as easily have been named polypropylene terephthalate since three methylene groups in a sequence is called propylene. Just as PET has two methylene groups as part of the polymer repeating unit and PBT has four, PTT has three. So it is fairly easy to predict the behavior of the material and its properties by interpolating between PET and PBT.
PTT is made by reacting terephthalic acid with propylene glycol (more properly 1,3-propanediol). Interestingly, it was developed in 1941 by the same two people, John Rex Whinfield and James Tennant Dickson, who first created PET. While the utility of PTT was recognized for use in fibers early on, the material was not commercialized because of the cost associated with producing propylene glycol. Shell Chemical began producing propylene glycol in larger amounts in the 1960s and in the 1990s the chemists at Shell applied a process known as hydroformylation to ethylene oxide to significantly increase the production of propylene glycol. This opened the door to commercial production of PTT. In fiber form it is trade named Corterra, and RTP Company markets a wide variety of PTT compounds for melt processing. Like PBT, it can be supplied either unfilled or with a variety of fillers and reinforcements.
These three chemistries that all fall into the general category of polyester are just the tip of the iceberg. Polyester chemistry is extremely versatile and many commercial materials contain polyester chemistry even if they do not bear a name that suggests such a connection. In our next installment, we will review some of those.
ABOUT THE AUTHOR: Michael Sepe is an independent materials and processing consultant based in Sedona, Ariz., with clients throughout North America, Europe, and Asia. He has more than 45 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 • mike@thematerialanalyst.com
Related Content
How to Optimize Injection Molding of PHA and PHA/PLA Blends
Here are processing guidelines aimed at both getting the PHA resin into the process without degrading it, and reducing residence time at melt temperatures.
Read MoreUnderstanding the ‘Science’ of Color
And as with all sciences, there are fundamentals that must be considered to do color right. Here’s a helpful start.
Read MoreThe Effects of Stress on Polymers
Previously we have discussed the effects of temperature and time on the long-term behavior of polymers. Now let's take a look at stress.
Read MoreThe Strain Rate Effect
The rate of loading for a plastic material is a key component of how we perceive its performance.
Read MoreRead Next
Tracing the History of Polymeric Materials: More on Polycarbonate
The industry has learned a lot about the advantages and disadvantages of polycarbonate during its more than 60-year history, and it would be difficult to imagine a world in which it did not exist.
Read MoreTracing the History of Polymeric Materials: How Nylons & Polyesters Connected
The history of nylons and polyesters are intertwined, and it takes some knowledge of chemistry to understand why.
Read MoreTracing the History of Polymeric Materials: PET
How PET evolved from a material for fibers and fabrics to a force in packaging.
Read More.jpg;width=70;height=70;mode=crop)