Tracing the History of Polymeric Materials: Nylon
The story of nylon, the first true engineering thermoplastic.
DuPont became involved in the cellulosics business when it acquired the rights to cellophane from the French company founded by Jacques Brandenberger. As innovative as cellophane was, it was a poor moisture barrier, a deficiency that was solved by William Hale Charch at DuPont in 1927. In that same year, DuPont embarked on a program to fund pure research in material science. This was a departure from how corporations up to this point had participated in scientific research.
Typically in the U.S., innovations had come from private entrepreneurs or from academia and then were adopted by manufacturing companies through technology transfer, acquisition, or licensing. To support this new approach to the development of materials, Wallace Carothers was hired away from Harvard and joined DuPont’s pure research effort at the Experimental Station in early February of 1928.
Carothers had focused on organic chemistry throughout his schooling and had become an instructor, first at the University of Illinois for two years, and then at Harvard. During the same time that Carothers was obtaining his advanced degrees, Hermann Staudinger was advancing his then- controversial theories that polymers such as rubber and cellulose were actually comprised of individual molecules of enormous size. He first proposed this in 1920 and published further on the topic in 1922, coining the term macromolecules.
Staudinger’s ideas were in conflict with the conventional wisdom regarding the unique properties of polymers. In 1926, when Staudinger took over the post he would hold for the rest of his professional career at the University of Freiburg in Germany, he was advised by his predecessor to abandon his idea of very large molecules, claiming that molecules with a molecular weight greater than 5000 did not exist and could not be synthesized. As we have noted previously, revolutionary ideas often evolve, and the persons historically recognized as the innovators very often are those who put the final pieces of the puzzle in place.
 The name "nylon" evolved from the search for a fiber that could produce "no-run" stockings.
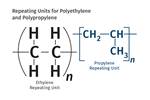
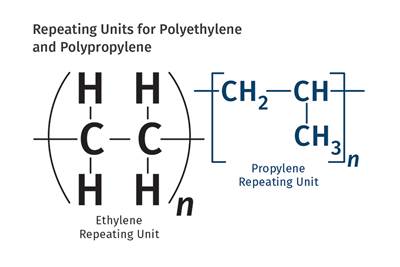
The name "nylon" evolved from the search for a fiber that could produce "no-run" stockings.The use of the word polymers, and the idea that they could be synthesized, can be traced back to Marcellin Berthelot, the French chemist who used catalysts like sulfuric acid to promote reactions with low-boiling-point compounds like ethylene and propylene, creating longer-chain hydrocarbons that he referred to as polyethylene. These molecules were still of relatively low molecular weight, perhaps a little over 200 g/mole. Later, the German chemist Emil Fischer created a material with a molecular weight of 4200, and the conventional wisdom was that this was the practical upper limit.
The use of the word polymers, and the idea that they could be synthesized, can be traced back to Marcellin Berthelot.
But Staudinger was relentless. In collaboration with the accomplished physicist Gustav Mie, Staudinger discovered polyoxymethylene (POM), also called acetal, in the 1920s. While POM would not be commercialized for another three decades due to problems with thermal stability, Staudinger was able to show that the size of the crystals formed by this polymer were much smaller than the individual molecules. In addition, Staudinger and Mie were able to produce fibers from the synthetic materials they were creating, showing that these materials could offer an alternative to natural fibers.
One of the people who was paying attention to Staudinger’s work was Wallace Carothers. Tasked with making synthetic materials that could be used in the form of fibers, Carothers and his team, working out of a laboratory in Wilmington, Del., that came to be called Purity Hall, set as an objective the creation of a material with a molecular weight that exceeded 4200. The purity of fundamental research did not last long. By the end of 1929, Carothers had nothing to show for his efforts to synthesize a high-molecular-weight material, and his immediate boss began to press for some practical results that could be commercialized. He did not have to wait long.
Early in 1930, while investigating compounds based on acetylene, a member of the Carothers team, Arnold M. Collins, isolated the liquid compound chloroprene, essentially chlorinated butadiene. In another example of accidental discovery, Collins set aside the unknown material for a period of time and when he returned to the test tubes, he found that the material had congealed into a solid that bounced when dropped. The chloroprene liquid had spontaneously polymerized into polychloroprene and was the first truly synthetic rubber. When crosslinked by vulcanization it became a useful material with better weatherability and resistance to grease and oil than natural rubber. Today the material is commonly known as Neoprene after being sold for a few years as Duprene.
Neoprene is created through free-radical addition polymerization, a relatively easy reaction to run. But it could not be turned into a fiber. The chemistry for developing synthetic fibers went the route of condensation reactions, a much more challenging process to control since it produces by-products that hinder the polymerization process. These developments started with the efforts of another member of the Carothers team, Julian Hill. In the same year that polychloroprene was synthesized, Hill finally broke the molecular-weight barrier by reacting a difunctional organic acid and a difunctional alcohol (a glycol) to produce a polyester with an average molecular weight of 12,000 g/mole.
The process of cold drawing would be instrumental in developing nylon.
In confirmation of Staudinger’s theories, Carothers observed that with the increased molecular weight came improved mechanical properties, especially ductility, which manifested as the ability to stretch the fibers considerably without breaking them. This allowed for the process of cold drawing. But the polyester that Hill created was not the same as the types of polyesters we use today. His material was too soluble in water to permit laundering and had too low a melting point for ironing of a fabric made from the fibers.

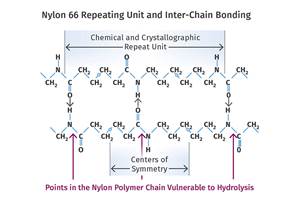
However, the process of cold drawing would be instrumental in developing the solution that would come five years later. After setting aside the effort to make a synthetic fiber for a period of time, Carothers returned to the problem in 1934, replacing the glycol with a difunctional amine. In that year, another member of the Carothers team, Donald Coffman, created a fiber based on an amino chemistry that solved all the drawbacks associated with the polyester. But the chemistry of the reaction was complicated, and Carothers and his team experimented with different variations. Within a year they had narrowed the field to two options, nylon 5/10 and nylon 6/6. Carothers preferred the nylon 5/10, but nylon 6/6 was selected because it was easier to prepare the intermediates from benzene, which was readily available.
The material was originally called Fiber 66 and was rapidly scaled up from a few pounds to 2000 lb. Paul Flory, a physical chemist who would later win a Nobel Prize for his work in polymers, assisted with the program by developing a strategy for stabilizing the tricky condensation reaction. Carothers himself developed the equation that describes the relationship between the degree of conversion of monomer to polymer and the final molecular weight of that polymer. In 1938, DuPont began construction of a plant in Delaware that had the capacity to produce 12 million lb of nylon fiber. Carothers would never see this accomplishment. Plagued by depression for most of his life, he committed suicide in Philadelphia on April 29, 1937, by ingesting cyanide.
In 1938, DuPont began construction of a plant in Delaware that had the capacity to produce 12 million lb of nylon fiber.
The polymer developed by Carothers and his team was the first of a large family of polyamides, the term properly used to describe these materials. There are many apocryphal stories regarding the origin of the word nylon as a description for the polymer. Today the naming process would likely be turned over to an advertising firm who would charge eight figures for the result. But it appears to have been a relatively clumsy process driven by the leaders of the DuPont chemical division who were presented with hundreds of proposals and eventually started with the term “norun” which then slowly morphed through several linguistic steps into nylon.
The focus during the initial decade of production was fiber, first for stockings, then during World War II for parachutes, tire cord, flak jackets, and mosquito netting, and then back to the fashion industry in 1945. But the properties were readily adaptable to other types of melt processing such as injection molding and extrusion as the material became the first true engineering thermoplastic.
In parallel with these developments, a series of materials were discovered and commercialized that employed the chlorine chemistry exemplified by Neoprene. These materials will be the focus of our next installment.
ABOUT THE AUTHOR: Michael Sepe is an independent materials and processing consultant based in Sedona, Ariz., with clients throughout North America, Europe, and Asia. He has more than 45 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 •mike@thematerialanalyst.com
Related Content
Resins & Additives for Sustainability in Vehicles, Electronics, Packaging & Medical
Material suppliers have been stepping up with resins and additives for the ‘circular economy,’ ranging from mechanically or chemically recycled to biobased content.
Read MoreWhat is the Allowable Moisture Content in Nylons? It Depends (Part 1)
A lot of the nylon that is processed is filled or reinforced, but the data sheets generally don’t account for this, making drying recommendations confusing. Here’s what you need to know.
Read MoreTracing the History of Polymeric Materials: Aliphatic Polyketone
Aliphatic polyketone is a material that gets little attention but is similar in chemistry to nylons, polyesters and acetals.
Read MoreScaling Up Sustainable Solutions for Fiber Reinforced Composite Materials
Oak Ridge National Laboratory's Sustainable Manufacturing Technologies Group helps industrial partners tackle the sustainability challenges presented by fiber-reinforced composite materials.
Read MoreRead Next
Tracing the History of Polymeric Materials: Commodity Resins
How the ‘Big 4’ commodity materials — PE, PP, PS, PVC — came to be.
Read MoreTracing the History of Polymeric Materials: Cellophane
How—and when—cellulose-based chemistry led to the discovery of cellophane.
Read More
.jpg;width=70;height=70;mode=crop)