Tracing the History of Polymeric Materials: Celluloid
In this series we’ll delve into a discerning look back into the history of our industry and how we all got here.
Periodically I get e-mails asking me if I have heard of particular historical events related to the plastics industry. One of the stories that gets a lot of attention is the one about John Wesley Hyatt, an American inventor credited with creating a material that is often referred to as the first plastic. The material was patented in 1869 under the name of Celluloid. The part of the story that seems to garner the most attention is the fact that Hyatt was awarded a $10,000 prize that had been put up by Michael Phelan, a master billiards player who had become concerned in the early 1860s about an ivory shortage and the impact this was having on the cost of the billiard balls.
The story is very appealing for a couple of reasons. First, it reinforces the idea that is very much ingrained into our industry that synthetic materials created through the genius of chemistry have displaced and improved upon materials derived from natural sources. The other factor is the size of the monetary award, equivalent to almost $200,000 today.
As usual, the story of the invention of Celluloid is much more complicated and relied heavily on previous accomplishments. And its introduction was accompanied by another noteworthy invention that has had a much greater impact on our industry than the material itself. And while the accomplishments associated with creating synthetic materials are primarily those of science, they are intertwined with the world of business, and as a result—because money is involved—with lawyers. This series of articles will be devoted to providing a more discerning look back into the history of our industry and how we all got here.
The world of synthetic materials was inspired by the world of materials that can be found in nature. The material that appears to have started the whole process was natural rubber, a substance derived from certain trees and chemically known as polyisoprene. The chemical structures for two different arrangements of the atoms in the molecule, known as isomers, are shown in the accompanying image.
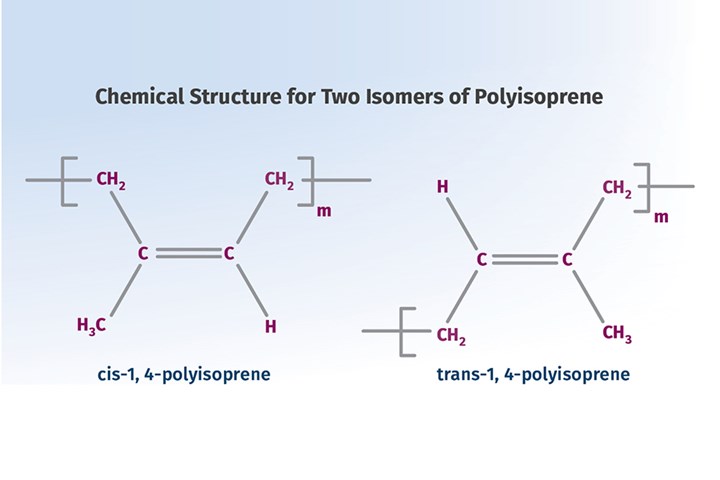
European explorers traveling to the Caribbean and Mesoamerica in the 16th and 17th century found civilizations that used this material to make solid balls as well as employing it to make fabrics waterproof. The existence of a solid ball made of a material with what today we would call elastomeric properties was a revelation to people from northern Europe who had only experienced balls made of an inflated bladder of leather. It was the cis-isomer that produced all of these products. We will get to the trans-isomer shortly.
The world of synthetic materials was inspired by the world of materials that can be found in nature.
A French explorer encountered a similar material when he traveled to Peru in the 1730s, and by 1751 the first scientific paper on this new material had been presented. But at this point the chemistry of the material was not well understood. In particular, the effect of temperature on the properties of the material created barriers to commercial use in Europe. Unlike the climates of Mesoamerica, where temperature fluctuations at a given elevation were relatively small, temperature changes in Europe from winter to summer were more significant. At cold temperatures the material became hard and brittle while the hot temperatures of summer made the material very soft and sticky. The most creative use for the product into the latter part of the 18th century was as an eraser of lead pencil marks from paper. The name rubber comes from this particular quality.
Progress in this era of chemistry was largely a matter of accidental discoveries brought about through trial and error. In 1820 two businessmen in very different fields independently discovered through just such accidents that polyisoprene was soluble in naphtha and turpentine. The dissolved rubber could then be applied to cotton to make waterproof clothing. This worked well as long the weather did not get too hot. When it did the coated fabrics would become sticky and lose their shape.
The temperature limits for the use of polyisoprene continued to be a problem until the 1830s and ’40s, when Charles Goodyear, using experimental methods just as random as those of his predecessors, stumbled upon two techniques that addressed first the high-temperature performance problems and then, three years later, the more well-known process of vulcanization that improved the properties of the material at low temperatures. Goodyear had no understanding of the chemistry behind the crosslinking process that dramatically improved the performance of the material. Even the term vulcanization was coined by a British competitor who figured out Goodyear’s approach and filed for patents in England while Goodyear was filing in the U.S.
Rubber compounding, the modification of the material properties through the addition of plasticizers and fillers, was still decades away. But the foundation for the world of polymers had been established. Interestingly, the native people of Mesoamerica had learned how to stabilize the properties of the rubber hundreds of years earlier by smoking the raw latex, presumably introducing in a less controlled but equally effective manner the nitrates and sulfur compounds needed to crosslink the material.
At the same time that court cases between Goodyear and his British counterparts were raging in the 1850s, a British surgeon practicing in southeast Asia observed the indigenous people in that part of the world extracting a sap from one of the trees that grew in that part of the world. They softened the material in hot water, and molded it into a variety of useful products such as tool handles and walking sticks. Named gutta percha after the species of tree that produced the sap, it is chemically the trans-isomer of polyisoprene.
This is an early and excellent illustration of the importance of isomerism in determining the properties of polymers, a principle we make extensive use of in modern polymer chemistry. The cis-isomer is amorphous and very sensitive to changes in temperature. This makes crosslinking necessary in order for the material to be useful. The trans-isomer is capable of crystallizing. Therefore, while it has the same subambient glass-transition temperature as the cis-isomer, it has useful solid properties above room temperature.
The discovery of gutta percha is an early and excellent illustration of the importance of isomerism in determining the properties of polymers, a principle we make extensive use of in modern polymer chemistry.
While gutta percha was another material that had been known and used by indigenous cultures for hundreds of years, in the hands the more goal-oriented Europeans, it was quickly adopted as an insulating material for underwater telegraph wires. In this respect it showed some similarities but also some important differences with the cis-isomer rubber. The non-polar structure of both materials makes them good electrical insulators. But the amorphous structure of rubber, even in its crosslinked form, resulted in a material that lacked chemical resistance to salt water. Gutta percha had the desirable electrical properties but at the same time was resistant to salt water as well as many other chemicals. This principle of improved chemical resistance arising from crystallinity is also well known in the world of polymers, and it made possible new applications very early in the history of our industry.
This also brings into focus another very important aspect associated with the use of materials: the relationship between the development of new chemistries and the invention of processing methods. While this material was used to coat electrical wire, the ability to do so was made possible by a very important invention: the extruder.
In our next installment we will continue the narrative of our progress toward Celluloid and its intersection with another very important development in processing.
ABOUT THE AUTHOR: Mike Sepe is an independent, global materials and processing consultant whose company, Michael P. Sepe, LLC, is based in Sedona, Ariz. He has more than 40 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 • mike@thematerialanalyst.com.
Related Content
New Entrant Heartland Polymers Stepping up as Reliable Supplier
Heartland Polymers’ new Alberta, Canada facility will produce 525 KTA propylene and 525 KTA polypropylene. It is expected to stabilize supply chains across the continent.
Read MoreUnderstanding the ‘Science’ of Color
And as with all sciences, there are fundamentals that must be considered to do color right. Here’s a helpful start.
Read MoreCommodity Resin Prices Flat to Lower
Major price correction looms for PP, and lower prices are projected for PE, PS, PVC and PET.
Read MoreThe Effects of Temperature
The polymers we work with follow the same principles as the body: the hotter the environment becomes, the less performance we can expect.
Read MoreRead Next
How Polymer Melts in Single-Screw Extruders
Understanding how polymer melts in a single-screw extruder could help you optimize your screw design to eliminate defect-causing solid polymer fragments.
Read MoreLead the Conversation, Change the Conversation
Coverage of single-use plastics can be both misleading and demoralizing. Here are 10 tips for changing the perception of the plastics industry at your company and in your community.
Read More
.jpg;width=70;height=70;mode=crop)
















.png;maxWidth=300;quality=90)