Enhanced Medical/Healthcare Materials to Debut at MD&M West
Polyplastics’ new POM series for drug contact/delivery applications and SABIC’s new PC copolymer family for medical devices and equipment housings among highlighted materials.
Expect to see debuts and expanded specialty portfolios of materials for a broad range of medical/healthcare applications at the upcoming (February 11-13) MD&M West in Anaheim, Calif. Among material suppliers to launch new products at the world’s largest medical design and manufacturing event are:
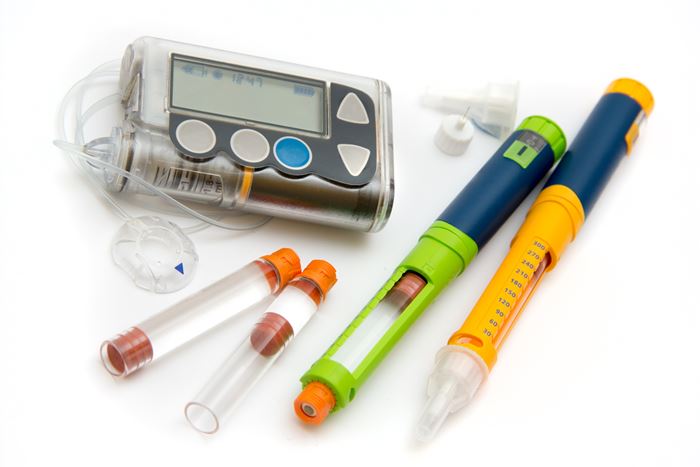
▪ Polyplastics (U.S. office in Farmington Hills, Mich.) will feature its new Duracon PM Series of POM (polyoxymethylene) for drug contact and delivery applications.
The first grade under the new series, PM09S01N, is reportedly finding growing acceptance in the market by delivering global medical and food regulatory compliance. This POM grade meets regulatory compliance requirements including ISO10993 and USP Class VI biocompatibility/cytotoxicity, FDA Drug Master File (DMF) and Device Master File (MAF), and EU 10/2011 and FDA food-contact 21 CFR 177.2470.
The new grade also adheres to strict quality management systems including conformity to VDI guideline, VDI 2017 medical-grade plastics. It also provides full traceability of processes and products, and production management based on GMP (Good Manufacturing Practices) principle. Polyplastics also reportedly provides uniform quality and global supply.
Polyplastics will also feature the latest for its Topas COC (cyclic olefin copolymer), a high-purity material for a range of medical applications which complements the PM series. A glass-clear and highly pure plastic, Topas COC boasts stiffness and barrier resistance, biocompatibility, and drug compatibility for wearables, drug delivery, medical devices, pharmaceutical blisters and trays, and diagnostics and microfluidics.
As with other major material suppliers in this arena, the company offers medical device manufacturers extensive data on the long-term reliability of its materials. Customized data on extraction, moldability, durability, slip and wear, and other key attributes is also available
▪ SABIC (U.S. office in Houston) will launch a family of PC copolymer resins that will have a new brand designation. The materials reportedly feature the exceptional chemical resistance needed to enhance the durability and resilience of medical devices and equipment housings. According to SABIC, its proprietary copolymer technology can help prevent premature part failure from environmental stress cracking (ESC) due to increasingly aggressive disinfectants, such as alcohols, peroxides and quaternary ammonium compounds, used to prevent hospital-acquired infections (HAIs). SABIC’s new portfolio includes amorphous and semi-crystalline materials that can serve as potential drop-in solutions in existing production tooling.
Also at the show, SABIC experts will address challenges and material solutions to help mitigate healthcare disinfectant exposure on February 11, from 3:30-4:15 p.m. PST in the Tech Theater. “Keeping Medical Equipment Clean and Durable: PC Copolymer Technology Innovations that Improve Chemical Resistance Against Hospital Disinfectants,” will be presented by business development manager Manish Nandi, SABIC Specialties, Americas, and senior business manager Nithin Raikar, SABIC Specialties.
Related Content
-
Scaling Up Sustainable Solutions for Fiber Reinforced Composite Materials
Oak Ridge National Laboratory's Sustainable Manufacturing Technologies Group helps industrial partners tackle the sustainability challenges presented by fiber-reinforced composite materials.
-
Lanxess and DSM Engineering Materials Venture Launched as ‘Envalior’
This new global engineering materials contender combines Lanxess’ high-performance materials business with DSM’s engineering materials business.
-
Volume Resin Prices Move in Different Directions
PE, PP, PVC, and ABS prices slump, while PS, PET, PC, and nylons 6 and 66 prices rise.