Mussels Inspire New Plastic
It can stretch without snapping and repair its own molecular bonds.
Nature’s inspiration for the development of new materials appears to never cease. Earlier this year, I reported on the continued evolution of biodegradable bioplastics, including those sourced or inspired by cassava root, proprietary starch formulations and natural fibers, as well as shrimp shells.
The shrimp development refers to man-made bioplastic chitosan being derived from the organic compound chitin, which extracted from shrimp shells, for the production of biodegradable shopping bags, as well as next-generation food packaging. Now mollusks have joined the ‘seafood’ crowd.
Mussels, in particular, have been the focus of research conducted by Megan Valentine, associate professor of mechanical engineering, at the University of California, Santa Barbara (UCSB), leading them to create a plastic that can stretch without snapping and repair its own molecular bonds, so it has potential for use in robot joints that lift heavy objects, or for packaging to protect delicate cargo from accidental falls.
Like other mollusks, mussels hang onto solid surfaces using an adhesive protein and tough fibers, which are extremely strong and can repair themselves when a few molecular bonds within them are broken. For a mussel, these stretchy yet strong fibers come in handy when a wave hits.
Valentine and her team created a plastic with the same properties as mussels by mimicking the chemistry the mussels use. Molecular bonds between iron and an organic compound called catechol make the material difficult to break or tear, while still allowing it to remain stretchy. The iron-catechol bonds dissipate energy from something hitting or stretching the material.
These “sacrificial bonds” break, but the overall structure stays intact, according to the researchers. Said Valentine, “It’s like a bike helmet: if you’re in a bike accident, the foam inside the helmet crushes and dissipates some of the energy. All that energy that would have gone into a skull fracture, instead goes into the helmet….In our case, instead of foam, we have this sacrificial bonding that protects the underlying polymer system.”
It is the iron-catechol bonds “sacrifice” that results in a material that can be stretched by 50%. Then, once the stress is removed, the bonds reform, making it reusable. Adding these bonds results in the plastic being 770 times stretchier and 58 times stronger than it is without them.
Noted Niels Holten-Andersen, associate professor of material science & engineering at the Massachusetts Institute of Technology (MIT), “Typically, there’s a trade-off: you can make a material harder to break but less stretchy, or easier to break and easier to stretch….But, by adding these mussel-inspired bonds, they’ve made it so that you don’t have to make that choice. A material that is both strong and stretchy could act as a shock absorber in packaging from fragile objects or be used to make body armor that repairs itself on a molecular level after taking a hit,” he added.
Valentine said the material could both find application in the joints of robotic arms that need to bear heavy weights while moving around, and potentially could be used to repair tendons in our joints.
Related Content
Cling Wrap Made from Potato Waste
Australia’s Great Wrap to expand into U.S. with home compostable cling wrap and its refillable dispenser made from recycled PET bottles.
Read MoreHow to Optimize Your Molds and Hot Runners for Processing Bioresins
Demand for bioresins is growing in molded goods, particularly as a sustainability play to replace fossil-fuel based materials, but these materials are not a drop-in replacement for traditional materials. Molds and hot runners need to be optimized for these materials.
Read MoreResins & Additives for Sustainability in Vehicles, Electronics, Packaging & Medical
Material suppliers have been stepping up with resins and additives for the ‘circular economy,’ ranging from mechanically or chemically recycled to biobased content.
Read MoreHow to Optimize Injection Molding of PHA and PHA/PLA Blends
Here are processing guidelines aimed at both getting the PHA resin into the process without degrading it, and reducing residence time at melt temperatures.
Read MoreRead Next
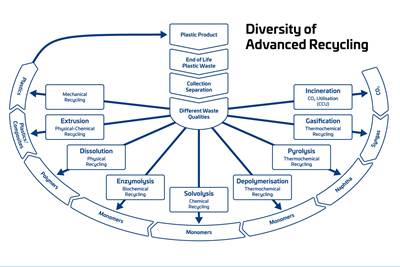
Advanced Recycling: Beyond Pyrolysis
Consumer-product brand owners increasingly see advanced chemical recycling as a necessary complement to mechanical recycling if they are to meet ambitious goals for a circular economy in the next decade. Dozens of technology providers are developing new technologies to overcome the limitations of existing pyrolysis methods and to commercialize various alternative approaches to chemical recycling of plastics.
Read MoreHow Polymer Melts in Single-Screw Extruders
Understanding how polymer melts in a single-screw extruder could help you optimize your screw design to eliminate defect-causing solid polymer fragments.
Read More