The Need for Generalists: Part 4
Solving problems generally requires distinguishing good samples from bad ones. But that can become clumsy when one person runs the test while another analyzes the data.
When I started my first test lab in 1986, I was the first and only employee. This was an invaluable experience because it meant that I fulfilled all the functions required in the lab. Setting up the equipment, learning the software, establishing the test methods, making samples, running the tests, analyzing the data, writing the reports, and maintaining the equipment were all part of the job description. It never occurred to me that it could be any other way.
Then one day I was working with an outside facility that had performed some tests that I could not do. I had noticed an anomaly in the raw data and had contacted the project engineer about calling up the files to review the anomaly. It was late in the afternoon, and the engineer explained to me that to access the data he needed to get into the software program for the instrument and this would have to wait until the next morning, when the technician responsible for operating that piece of equipment came in.
I was stunned. The idea that an engineer responsible for reviewing and reporting on results from an instrument could not, essentially, turn on the machine, was foreign to me and at the same time a bit troubling. But as I have worked with a larger number of established facilities over the years, I have come to realize that this is typical. The functions in the lab have been compartmentalized. The technicians operate the equipment and provide the raw results to a project engineer. The test methods may be developed by highly skilled chemists or mechanical engineers before they are handed off to the technicians for execution of the tests, but the actual sample preparation and running of the instruments is the responsibility of the technicians.
Sometimes these functions can be parceled out in a remarkably narrow fashion. I visited a lab in the early 1990s where one technician ran the DSC, one ran the TGA, one ran the FT-IR, etc. That is all each person did. Not only was it very inefficient, it was mind-numbingly boring for the people doing the work. That lab has since closed, for obvious reasons.
In a typical lab today, the project engineer receives the raw data from the technician or technicians and organizes it into a report that is supposed to provide a solution to whatever problem has been posed by the client. And here is where it gets interesting. Solving a client problem involves identifying characteristics that distinguish “good” samples from “bad” samples. Consequently, the person reviewing the raw data should be looking for differences between samples that can then be correlated to the reported differences in function or performance observed by the client. When the task of preparing the samples and running the tests is given to one set of people and the analysis is performed by a separate group, the chances increase that important clues will be missed.
Here is an example. Various PE tubes were provided for an evaluation of composition in an effort to account for localized brittle behavior in some of the tubes.
The PE was filled to a reported nominal level of 40%, and the density of the specified PE was 0.964 g/cm3. Two tests that come under the general heading of thermal analysis are essential in characterizing a filled semi-crystalline polymer. Differential scanning calorimetry (DSC) is used to evaluate the composition and crystallinity of the polymer by measuring the temperature range over which the polymer melts and the energy associated with that melting process. The other technique is thermo-gravimetric analysis (TGA), a method that heats a sample at a controlled rate and monitors the manner in which the material decomposes and loses mass. The temperature and rate at which this occurs and the material that remains at the conclusion of the test provide additional information about the condition of the polymer and also quantify the inorganic content in the material. Both of these tests provide information that has a direct bearing on the properties of the parts made from the material.
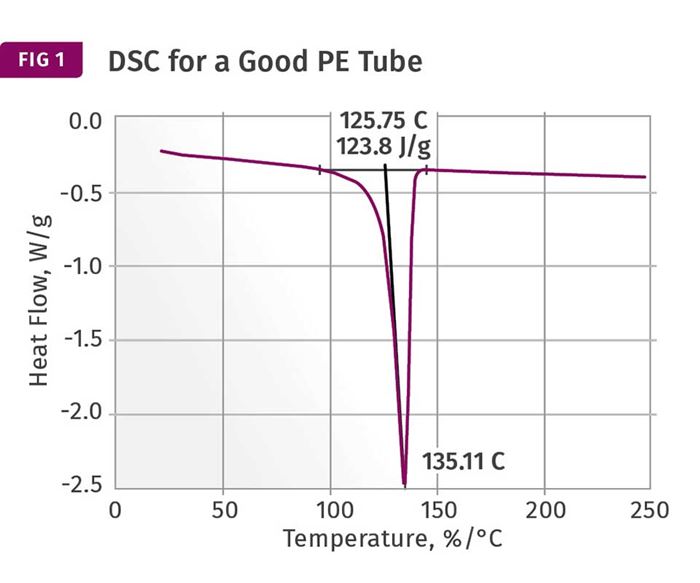
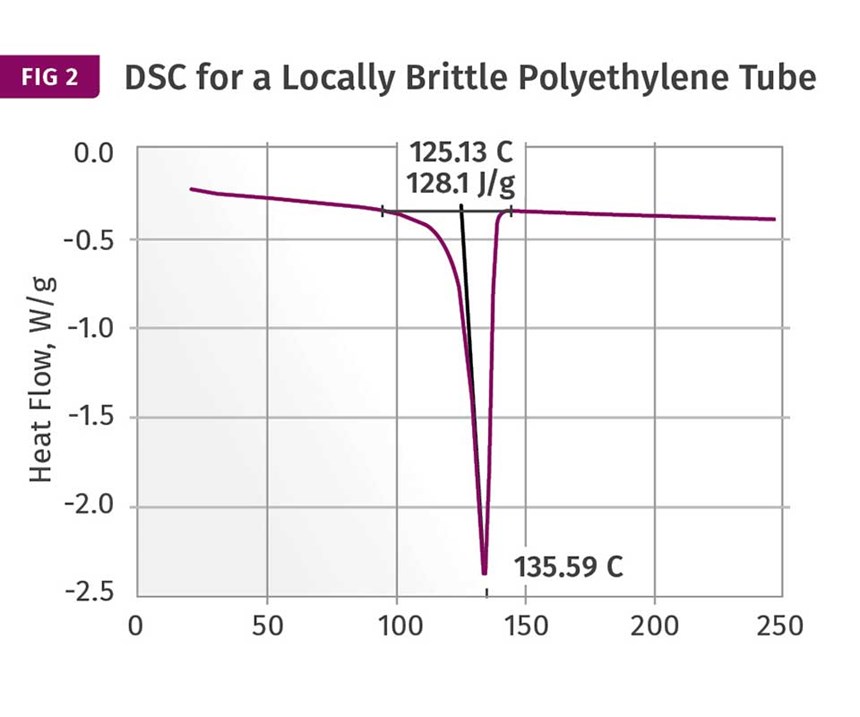
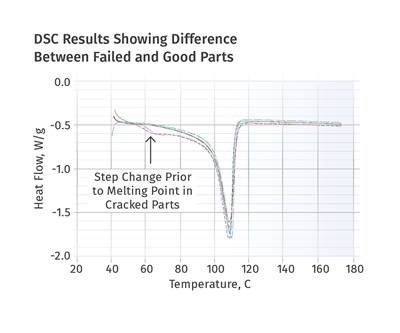
Figures 1 and 2 show DSC scans for samples taken from two tubes, one considered to be good and the other exhibiting localized brittle behavior. The results for both samples show a sharp melting event with a peak near 135°C (275°F) and heat of fusion values that are comparable, between 124 and 128 J/g. The higher heat of fusion is associated with the brittle sample. It might be tempting to point to this as a key data point, since a higher heat of fusion can be associated with a higher degree of crystallinity. This, in turn, can result in a material that is stronger and stiffer, but less ductile. But an analyst with experience knows that these values are much lower than normal for HDPE, and the reason for this reduction is the presence of the filler. This filler is inorganic and therefore does not undergo a phase change the way the polymer does.
But since the heat of fusion is given as an energy per unit mass of the whole sample, the presence of the filler dilutes this value in proportion to the amount of filler that is in the compound. Since the stated nominal filler content is 40%, we can readily calculate that the heat of fusion of the HDPE itself is slightly above 200 J/g, a value much more in keeping with the specified density of the polymer. In addition, since the amount of filler influences the heat of fusion, the small difference between the two samples may be attributable to small fluctuations in the filler content. So the picture cannot be complete until we have the TGA results.
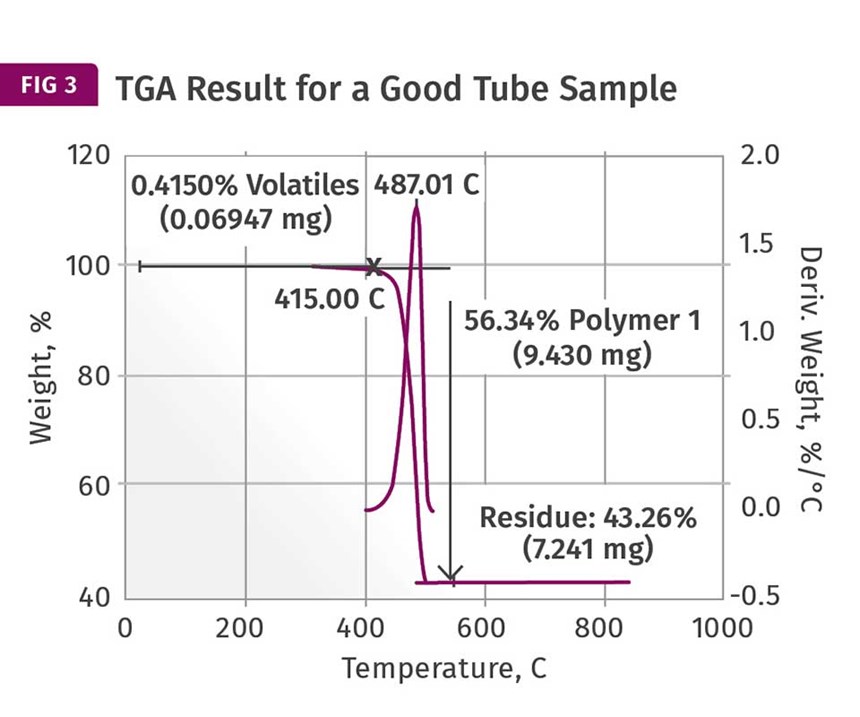
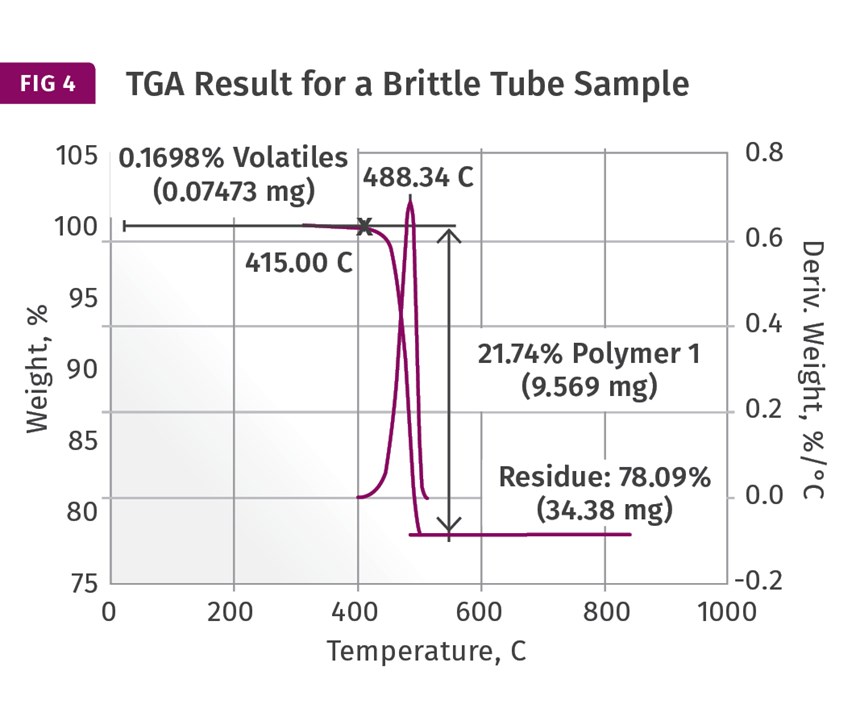
These are provided in Figs. 3 and 4. These show the weight loss and weight- loss rate as a function of temperature. In the good sample the PE decomposes, leaving a non-combustible ash content of just over 43%. This is reasonably close to the nominal specification of 40% inorganic filler. However, the result for the brittle tube shows a filler content of over 78%! This should immediately get the attention of someone involved in the analysis. I maintain that the impact of this finding is best understood when the person running the test is also the person evaluating the data. But too often the initial result is simply passed along and the appropriate attention is not given to the finding. The dots are not connected.
First, a filler content that is nearly double that of the nominal specification has a potentially important connection to the observed brittle behavior. In addition, the high filler content finding by TGA does not correlate well to the DSC result. If the DSC sample also contained this elevated level of filler, the heat of fusion would have been much lower because there would have been much less polyethylene in the sample. The fact that the DSC results agree well between the samples and the TGA samples do not suggests that the high filler content is a local condition within the tube and not a deviation in the composition of the entire batch or even the entire individual part from which the sample was taken. It potentially represents poor homogeneity within the compound.
Verification of the result demands more tests—at minimum a second TGA, and ideally an ash test that allows for an evaluation of a larger sample. But first the results must be understood well enough so that these tests are ordered and the project can be complete. Unfortunately, in many cases the results are simply reported without highlighting the anomalies and their potential significance to the problem. If the client does not have a background in analytical testing, which is usually the case, the answer to the problem will go unnoticed even though it is in plain sight in the data.
ABOUT THE AUTHOR Mike Sepe is an independent, global materials and processing consultant whose company, Michael P. Sepe, LLC, is based in Sedona, Ariz. He has more than 40 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 • mike@thematerialanalyst.com.
Related Content
Redesigned Laser Scanner Boasts Improved Accuracy, Resolution, Versatility, and Efficiency
Nikon Metrology’s new LC15Dx is ideal for efficient measurement and inspection of manufactured components including intricate plastic parts.
Read MoreHow Inline Vision Inspection Can Minimize Scrap in Molding
Once viewed by injection and blow molders as a necessary evil, machine vision technology today can continuously monitor and improve production while reducing costs.
Read MoreRobust Rotational Viscometer
Versatile viscometer line said to offer robust measurement, repeatability and reporting.
Read MoreHow to Improve Quality with Offline Inspection and Analysis
Automated sample testing with a light table detects the smallest contamination in flakes, micro granulates and sample test sheets.
Read MoreRead Next
The Need for Generalists, Part 2
Problem-solving requires a team that combines people having academic credentials with others who have hands-on experience.
Read MoreThe Need for Generalists: Part 1
Even companies with seemingly unlimited resources can have problems identifying root causes of problems. Maybe they have too many specialists and not enough generalists.
Read MoreThe Need for Generalists: Part 3
In failure analysis, there is a tendency to gravitate to a few common test protocols. But this approach can result in a mismatch of techniques to the problem.
Read More.jpg;width=70;height=70;mode=crop)