Canon Molds New Market in Medical Contract Manufacturing
Expanding more into medical product development and contract manufacturing, Canon Virginia finds success in projects that exploit the long-honed expertise of its parent company in areas like printing and imaging with demanding manufacturing requirements.
At Plastec West, the company showcased the newest example of this synergy with the blinq pediatric vision scanner—an FDA-cleared device to help detect micro-strabismus and amblyopia (lazy eye) disorders in children—which Canon will now manufacture for REBIScan Inc.
“Canon is known for imaging so how can we use that with the medical device industry,” explained Wayne Daniel, director business development Canon Virginia. “How can we incorporate our current technologies?”
REBIScan had been manufacturing the first-generation of the blinq, and its cooperation with Canon presented an opportunity to advance the technology. “We’ve leveraged again the optics side of it—we have some knowledge in that arena—and really we want to look at this as a partnership,” said Canon’s Rhonda Bunn. “So what does the next generation look like; how do we get involved on the engineering side, on the testing side, on the quality side and leveraging our other know how to really help the next level of product development.”

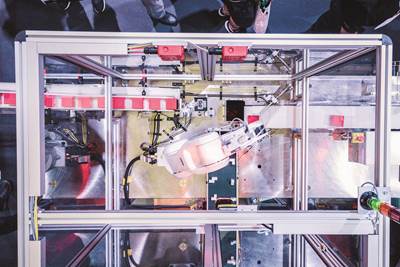
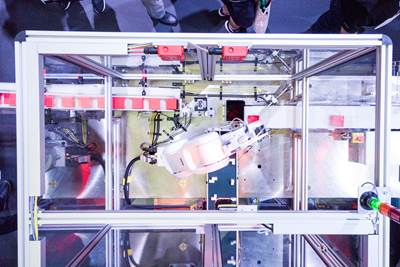
Manufactured by Canon Virginia, the blinq pediatric vision scanner from REBIScan Inc. is the latest product made by Canon’s growing medical contract manufacturing unit.
Ron Kurz, senior director and operations manager of business development at Canon, noted that the company’s insistence on vertically integrating all of its own production develops skills it can then bring to bear on its customers’ projects. “We acquire manufacturing knowhow that we can share across our factories,” Kurz said. “So we’re using all that know how that we’ve gained throughout the Canon group to support, not only medical products that we build for Canon, but now medical products that we’re building for third parties.”
Kurz said Canon partnered on the blinq project for several reasons. REBIScan came to Canon with some “optics challenges” related to the previous manufacturing process and product design that were related in part to the complex optical path within the device.
“Dust on optics, manufacturing know how, alignment, all those are very critical,” Kurz said. Part of Canon’s initial discussion was to have REBIScan tour its manufacturing site and let it see how Canon handles manufacturing optics for its own products. “We gave them a confidence level, basically, when they left here that we could do their product,” Kurz said. Certainty in its capabilities was instilled as Canon used Plastec to make a partnership announcement.
Room to Grow Into More Medical
At the moment, Canon’s focus going forward in the medical market is on devices classified as Class II by the U.S. Food and Drug Administration (FDA). FDA determines the classes on the basis of risk to the patient or user, with Class III being the highest risk. Canon is not currently looking at Class III business. “No orthopedics, no implantables, nothing like that,” Kurz said. Its medical focus, for the moment, comes in electromechanical parts and applications requiring complex injection molding and tooling, as well as significant intellectual property.
At its Canon Virginia facility, the company has 30,000-ft2 of dedicated space for medical product development. There is room to double that as needed, and it currently includes an ISO 7 cleanroom of 1000 ft2, with infrastructure in place for six additional individual cleanrooms. In addition, the company can offer mobile cleanrooms that install over an injection molding machine, creating a cell equivalent to ISO 7. More and more of that space could see use in medical contract manufacturing as the perception of Canon changes.
“One of the big things is there’s still the misconception out there that people just think we’re a camera and printer company,” Daniel said. “One of the things we communicate at shows is, ‘Yes, cameras and printers and copiers are a core business for us, however to manufacture all of those it takes all of these components.’”
Related Content
As Currier Grows in Medical Consumables, Blow Molding Is Its ‘Foot in the Door’
Currier Plastics has added substantial capacity recently in both injection and blow molding for medical/pharmaceutical products, including several machines to occupy a new, large clean room.
Read MoreWisconsin Firms Unite in Battle Against Covid
Teel Plastics opened new plant in record time, partnering with AEC & Aqua Poly Equipment Co. to expand production of swab sticks to fight pandemic.
Read MoreKrones Acquires Netstal
Krones adds PET preform injection molding to its bottle blowing and filling capabilities, as well as cap molding and expansion into medical, food and other markets.
Read MoreRecord Reshoring Rates in 2022
Reshoring and foreign direct investment (FDI) in the third quarter marked their highest ever level, eclipsing the previous record set in the second quarter of 2022.
Read MoreRead Next
Canon Virginia Graduates First Class of Apprentices
President Obama declared this week National Apprentice Week and in honor of that, employers across the country will host open houses to highlight the significant value of apprenticeships in our economy.
Read MoreNew System Runs Two Molds in One Press Simultaneously
Shuttle-mold system is one of several eye-popping innovations that Canon Virginia brought to NPE2018.
Read MoreInnovations in Molding, Machine Texturing, Micro-Fluidics and More at Canon Virginia Booth
Multi-Mold System shuttles molds in and out to run two separate tools in one press simultaneously.
Read More












.png;maxWidth=300;quality=90)