FDA Grants Formlabs Emergency Use Authorization for 3D-Printed Ventilator Component
Formlabs has the capacity to produce up to 3,000 3D printed adapters a day and is now shipping the adapters to hospitals for critically ill COVID-19 patients.

3D printed T piece adaptor. Credit: Northwell Health.
Formlabs has received emergency use authorization (EUA) from the U.S. Food and Drug Administration (FDA) to print bi-level positive airway pressure (BiPAP) adapters designed by Northwell Health, New York's largest healthcare provider. Formlabs is reportedly the first 3D printing manufacturer to receive an EUA and is now shipping these adapters to hospital systems throughout the U.S. to combat the shortage of ventilators and provide life-saving treatment to COVID-19 patients.
The 3D printed adapters convert BiPAP machines, typically used for patients suffering from sleep apnea, into functional invasive mechanical ventilators. The key component to converting the BiPAP machine is a small, plastic T-shape adapter, which is produced on 3D printing machines in hospitals across the country. Formlabs will also produce these adapters at its FDA-registered headquarters in Somerville, MA to distribute to hospitals and government systems throughout the United States.

Formlabs will allocate 150 3D printers at its headquarters towards printing these adapters, enabling the company to print up to 3,000 parts per day once production is fully ramped up.
“Prior to the COVID-19 pandemic, the FDA had only authorized a handful of EUAs over a 30 year period,” said Max Lobovsky, CEO and co-founder of Formlabs. “Formlabs’ EUA for BiPAP adapters signifies the need for these components and 3D printings' unique ability to fill that need. 3D printing enables rapid iteration and prototyping of new, innovative medical equipment, while expediting the production process, shortening supply chains, and allowing for localized manufacturing. Hospitals around the country can also use Formlabs’ printers to create these adapters locally under their own practice of medicine, meaning printing the adapters at scale in the hardest-hit areas is as easy as uploading a design and pressing print.”
Northwell Health has been a key partner of Formlabs throughout the COVID-19 pandemic. The hospital system began working with Formlabs in 2018 and that partnership has led to the creation of 3D printed test swabs, in collaboration with the University of South Florida Health, and now 3D printed BiPAP adapters. Northwell designed these adapters to provide life-saving care to hospitalized COVID-19 patients during the peak of the virus in New York City.
After using these adapters on a number of patients in their New York City ICUs during the peak of the virus in the city, Formlabs will now print these adapters at scale to aid hospitals in other hard-hit cities.
Back in March, I talked with Dávid Lakatos, Formlabs' Chief Product Officer and he mentioned how the company was looking to work with the medical community:
“Formlabs is looking for ways to help the medical community address the overall COVID-19 epidemic. We have many customers in the healthcare space already using Formlabs products for COVID-19 related projects, and recently launched the Formlabs Support Network for COVID-19 Response. This is an initiative to match Formlabs customers who are willing to use their printers to support healthcare needs with projects that could use their assistance.”
For more information on Formlabs response to COVID-19 visit, formlabs.com/covid-19-response. Those looking to volunteer their time and 3D printers to help combat COVID-19 can find more information here.
Related Content
Custom Molder Manages Growth on Several Fronts
Adding people, plants and machines, expanding capabilities in LSR, high-tonnage presses, automation and 3D printing—EVCO Plastics maintains momentum through challenging times.
Read MoreDaimler, OMIC Evaluate Wire-Fed DED for Moldmaking
3D printing a core and cavity on machine from Gefertec, followed by machining, allowed for a complete mold tool to be produced in three days.
Read MoreKraussMaffei Launches Two Additive Manufacturing Lines at K 2022
Long established in injection molding, extrusion and polyurethane reaction process machinery, 184-yr-old KraussMaffei prepares to enter the industrial additive manufacturing market.
Read More420 Stainless Steel Now Qualified With TrueShape 3D Printing Technology
NPE2024: Mantle's Additive Manufacturing Technology is Designed for Precision Tooling
Read MoreRead Next
3D Printing Helps Keep Production Going During the Coronavirus Crisis
Formlabs and Essentium both discuss how their companies are responding to the COVID-19 crisis and how 3D printing can help eliminate potential supply chain disruption.
Read MoreOhio Molder Joins Statewide Consortium to Combat Covid-19
Pragmatic Manufacturing put its new Wittmann Battenfeld molding cells to work as part of an effort to produce and deliver more than 1 million face shields for front-line health care workers in Ohio in just five weeks.
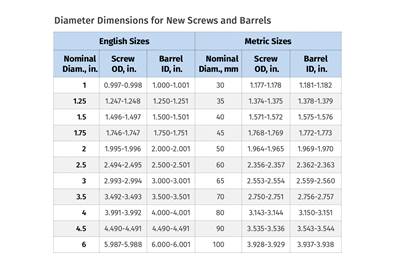
Read MoreTroubleshooting Screw and Barrel Wear in Extrusion
Extruder screws and barrels will wear over time. If you are seeing a reduction in specific rate and higher discharge temperatures, wear is the likely culprit.
Read More