FibreTuff Starts Up Compounding of Cellulose-Based Biomaterial with a Focus on Medical Devices
The facility will manufacture FibreTuff PAPC filaments and powders for 3D printing and compounds for molding of Class I and II medical devices for spine, trauma and sports medicine.
FibreTuff, West Unity, Ohio, held an open house on July 31, for the launch of compounding operations to manufacture cellulose based biomaterial for the 3D printing and molding of Class I and II medical devices for spine, trauma and sports medicine. The company expects to hire about 20 people over the next three years as companies across the U.S. begin to use FibreTuff PAPC filaments and powders for 3D printing.
Said company founder Robert Joyce, “This is innovative. This is disruptive to the medical devices market…The market is there, the opportunity is there, and we are adding more strategic partners as we grow.”
FibreTuff’s PAPC filament can be used in 3D printers without the odors traditionally associated with the printing process. In addition, the costs to those printing medical devices, such as cervical spacers and implants is about 30 percent less than other products in the marketplace.
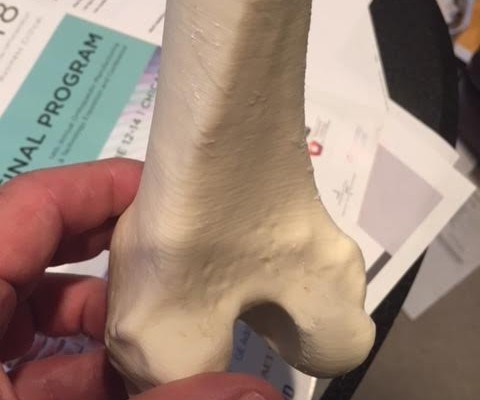
According to COO Ted Wolkowski, a key advantage is that it’s ‘radio opaque,’ which means it can be seen by an x-ray. “It doesn’t require any additives - like the current products on the market - to be detected by x-ray.” Yet other distinguishing characteristics of the FibreTuff filament are that it will not dissolve inside the body, it has passed USP Class VI testing performed by NAMSA for implantation and its weight and composition are similar to actual bone.
The cellulose based biomaterial branded as PAPC by FibreTuff, will be manufactured in West Unity and delivered to customers in the form of compounded pellets, filament and bar stocks for CNC machining, injection and extrusion processes. Customers from Boston to San Francisco are already using FibreTuff to produce bone replacements, mesh and temporary implants, and eventually, spinal spacers for orthopaedics.
FibreTuff intends to work with hospitals, colleges and universities, and medical device manufacturers to develop a new way to deliver education and functional tools and models to the medical market, explains Joyce.“The 3D bone replacements made with FibreTuff PAPC are so realistic, medical students can use them instead of animal cadavers for essential practice, such as sawing, screwing, cutting and laser cutting.”Related Content
-
How Additive Manufacturing Can Help, not Hinder, Injection Moldability of New Designs
Four cost drivers—design for moldability, mold-base size, internal componentry, polish/custom finishing—dictate the financial and processing success of a molded part design. Learn how 3D printing can assist this process, while also understanding its potential pitfalls.
-
Getting into Plastics Additive Manufacturing? Avoid these Six Common Errors
There are a lot of 3D printing technologies out there, and it’s not uncommon for processors new to additive manufacturing to get tripped up. Here are some typical snafus, along with advice on how to avoid them before you start making parts.
-
Medical Manufacturer Innovates with Additive Manufacturing and Extrusion Technology Hubs
Spectrum Plastics Group offers customers two technology hubs — one for extrusion, the other for additive manufacturing — to help bring ground-breaking products to market faster.