A Processor’s Most Important Job, Part 4: Mold Temperature
Engineering polymers require higher mold temperatures to achieve their ideal structure. The temptation to turn down the mold temperatures can hurt part performance.
This is part four of an eleven-part series focused on the most important job of a processor. To read the rest of this series, click these links:
part one, part two, part three, part five, part six, part seven, part eight, part nine, part ten, part eleven.
Generally, higher performance in polymers is associated with the ability of a material to withstand exposure to elevated temperatures. In semi-crystalline polymers, two important transitions characterize this capability: The glass-transition temperature (Tg) and the melting point (Tm). Polypropylene, which we discussed at the end of the last article, is generally considered to be a commodity material with a Tg that is subambient, near 0°C, and a Tm for most grades at 320-329°F (160-165°C).
This temperature difference between the two transitions is typical for most semi-crystalline materials. Aliphatic nylons such as nylon 6 and nylon 66, and semi-crystalline polyesters such as PBT and PET, form the next tier on the performance ladder, with Tgs of 131-194°F (55-90°C) and melting points of 437-500°F (225-260°C).
Unlike PP and polyethylene, which can be run at relatively low mold temperatures, these engineering polymers require higher mold temperatures to achieve their ideal structure. It is with these materials that the temptation to turn down the mold temperature can become problematic for part performance.
As we go up the performance scale to SPS, PPS, partially aromatic nylons—sometimes referred to as PPA, and very high-performance materials such as PEEK, the stakes get higher. These materials have Tg’s that exceed the boiling point of water (230-302°F). Therefore, maintaining mold temperatures high enough to allow these materials to crystallize to a satisfactory level requires the use of pressurized water, oil or electric cartridges. Not all processors are willing to adopt these technologies. But at this high-performance level, the consequences of failing to achieve the mold temperatures required to promote adequate crystallinity become especially severe.
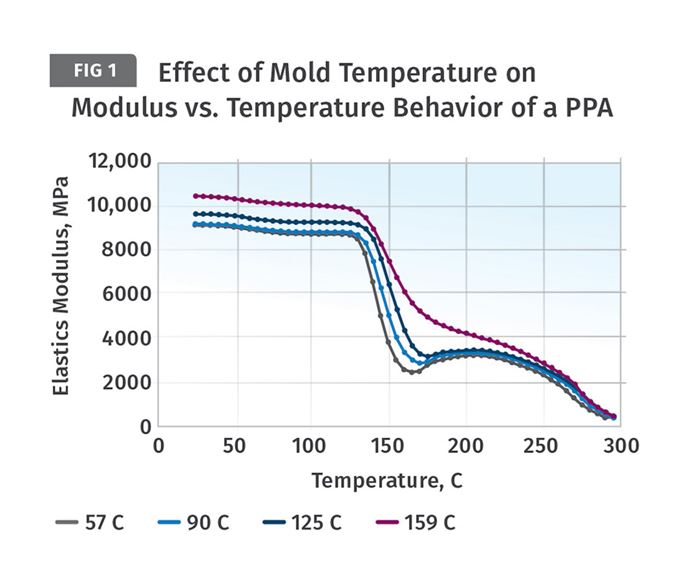
Figure 1 shows the behavior of test specimens molded using the four different mold temperatures noted in the legend: 135°, 194°, 257° and 318°F (57°, 90°, 125°, and 159°C). The material partially aromatic nylon (PPA) reinforced with 35-percent glass fiber. Specimens from each sample group were tested using dynamic mechanical analysis (DMA) to evaluate the temperature-dependent behavior of the material. This is provided as a plot of the elastic modulus of the material as a function of temperature. The different levels of crystallinity that the polymer was able to achieve at these various mold temperatures are evident from the response of each sample.
Know How Materials: For more insight on materials.
Notice that the initial modulus of the material trends upward with increasing mold temperature. The modulus of the sample molded at 57°C is approximately 9,000 MPa (1,300 ksi) while the sample molded at 159°C has a room-temperature modulus of 10,350 MPa (1,500 ksi). This is a 15-percent improvement. The largest increase occurs between the samples molded at 125°C and 159°C. A much greater difference in performance is evident as the test temperature increases. As the temperature of the tests reaches 266°F (130°C) the modulus of all four samples begins to decline rapidly. This is the onset of the glass transition. An exact measurement of the Tg for this material gives a value of 293°F (145°C).
Semi-crystalline polymers exhibit a significant decline in modulus as they pass through the glass transition. When polyamides (nylons) are filled with this level of glass fiber it is expected that the material will retain approximately 50 percent of its room-temperature modulus above the Tg. This is exactly what is observed for the specimen molded at the highest mold temperature. The samples molded at the lower mold temperatures exhibit progressively larger modulus reductions as the mold temperatures used to produce the samples decline. At the lowest mold temperature, the modulus decline is nearly 75 percent. This will have implications for long-term performance characteristics such as creep resistance and fatigue resistance, even at temperatures below the glass transition.
It is unfortunate that some suppliers of high-performance semi-crystalline materials tell molders that full crystallization is only important if the part is intended for use at temperatures above the glass transition. This is not at all true, something we will address in a later article.
Another notable difference between the sample molded at the highest mold temperature and the other three samples can be observed at the conclusion of the glass transition. The part molded at the highest mold temperature enters a slowly declining plateau region between the end of the glass transition and the onset of the melting point near 572°F (300°C). This is typical behavior for a properly crystallized polymer. The other three samples show another symptom of incomplete crystallization. As soon as the glass transition is complete, the modulus begins to increase, even though the temperature is increasing. This is caused by the formation of crystals that were intended to be produced during the molding process. But because these mold temperatures are too low to allow for a complete level of crystallinity, the shortfall has to be made up in the solid state once the mobility needed to form crystals has been re-established. This upturn in the modulus is also associated with the previously discussed dimensional changes that always accompany crystallization.
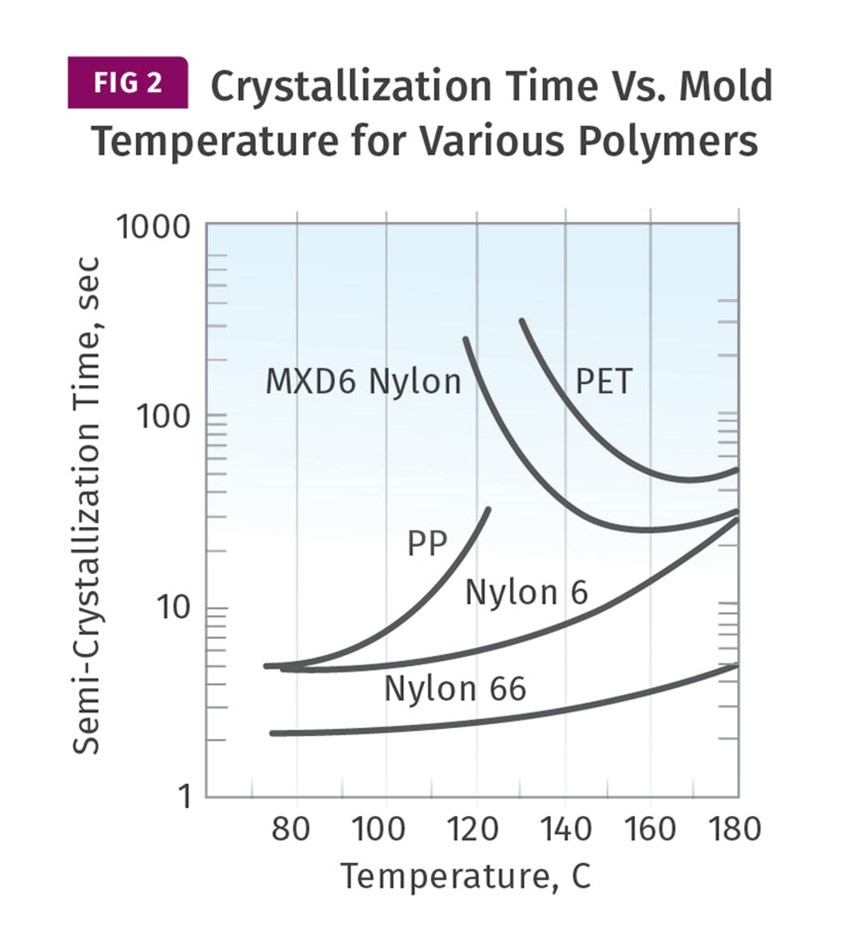
It is not a coincidence that the dividing line between the properly crystallized sample and the ones that fall short is the glass transition temperature of the polymer being molded. Maintaining a temperature above the Tg provides the molecular mobility needed to form crystals. Figure 2 shows the relationship between mold temperature and the rate of crystallization for various materials.
Some suppliers of high-performance semi-crystalline materials tell molders that full crystallization is only important if the part is intended for use at temperatures above the glass transition. This is not at all true.
Three of the profiled materials—PP, nylon 6 and nylon 66—are materials with glass-transition temperatures below the temperature range shown in the graph. The PP, with a Tg of approximately 0°C, will show only a marginal increase in crystallinity at higher mold temperatures, while crystallization time increases significantly, slowing the cycle time. The nylons, with Tgs of 65-70°C, will reach “full” crystallization using mold temperatures of 90-100°C, and it is evident that in this temperature range the crystallization time is essentially constant. As it rises above this point, the crystallization time slowly increases, once again extending cycle time.
The Polymers Behavior
The PET evaluated here is a non-nucleated material, so the behavior documented here is not typical of the highly filled semi-crystalline grades used for injection molding. The MXD6 is one of the partially aromatic nylons that we have been referring to. These materials, and others like them, such as PPS and PEEK, display a much different behavior than the polypropylene and the aliphatic nylons. With these materials, the rate of crystallization decreases significantly once the mold temperature declines below a certain point that is related to the Tg of the polymer. The effect is considerable.
For the MXD6 the crystallization time is 25 seconds at a mold temperature of 160°C, while it increases to 180 seconds at a mold temperature of 120°C. For these materials, not only does the lower mold temperature prevent the achievement of the desired level of crystallization, it increases the time required to reach an ejectable modulus, which in turn increases cycle time. Understanding the consequences of changing the mold temperature requires knowledge of the specific polymer being processed.
In our next article, we will look at a semi-crystalline polymer that does not follow the rule that maintaining a mold temperature above the Tg is the only requirement for achieving appropriate crystallinity.
About the Author
Michael Sepe
Michael Sepe is an independent, global materials and processing consultant whose company, Michael P. Sepe LLC, is based in Sedona, Arizona. He has more than 40 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting and failure analysis. Contact: 928-203-0408 • mike@thematerialanalyst.com.
Related Content
Commodity Resin Prices Flat to Lower
Major price correction looms for PP, and lower prices are projected for PE, PS, PVC and PET.
Read MoreFundamentals of Polyethylene – Part 6: PE Performance
Don’t assume you know everything there is to know about PE because it’s been around so long. Here is yet another example of how the performance of PE is influenced by molecular weight and density.
Read MorePrices Up for PE, PP, PS, Flat for PVC, PET
Trajectory is generally flat-to-down for all commodity resins.
Read MoreFormulating LLDPE/LDPE Blends For Abuse–Resistant Blown Film
A new study shows how the type and amount of LDPE in blends with LLDPE affect the processing and strength/toughness properties of blown film. Data are shown for both LDPE-rich and LLDPE-rich blends.
Read MoreRead Next
A Processor’s Most Important Job, Part 3: Unintended Consequences
Processors are often expected to compensate for ill-advised decisions made earlier in the product-development process. In the case of shrinkage, one of the most common ‘fixes’ is to simply reduce the mold temperature.
Read MoreA Processor's Most Important Job, Part 5: POM Polymers
Using a mold temperature above a polymer’s Tg ensures a degree of crystallinity high enough to provide for dimensional stability, even if the part must be used at elevated temperatures. But POM is an exception. Why?
Read MoreAdvanced Recycling: Beyond Pyrolysis
Consumer-product brand owners increasingly see advanced chemical recycling as a necessary complement to mechanical recycling if they are to meet ambitious goals for a circular economy in the next decade. Dozens of technology providers are developing new technologies to overcome the limitations of existing pyrolysis methods and to commercialize various alternative approaches to chemical recycling of plastics.
Read More.jpg;width=70;height=70;mode=crop)