Tracing the History of Polymeric Materials: The Differences Between Nylons & Polyesters
In many respects, nylons and polyesters appear to be interchangeable. But there are interesting differences in the properties of these two families that arise from their chemical structures.

Nylons and polyesters have overlapping properties, and each has its pros and cons. Careful comparison is required to determine the optimal choice for any particular application. (Photo: Domo Chemicals)
The history of polyesters and nylons is intertwined. Polyesters were created first but initially abandoned by their inventor, DuPont, in favor of nylon. Yet, once polyester chemistry was enhanced by other researchers using aromatic acids in making the polymer, the material became commercially viable and the rights to the resulting product were purchased by DuPont in the 1940s and commercialized by 1950, becoming a major pillar in the company’s polymer product offering.
Because polyester and nylon materials were developed at the same time using similar chemistries, the applications into which they were placed often overlapped. In many respects the materials appeared to be interchangeable. Both, for example, were quickly adopted as synthetic fibers to replace natural fibers such as silk, wool and cotton.
But there are interesting differences in the properties of these two families that arise from their chemical structures. The accompanying figure shows the functional amide and ester groups that characterize these materials. In most commercial nylons, short carbon chain segments extend from the amide group. The length of these chain segments depends upon the type of nylon, with short segments associated with materials like nylon 46 and nylon 66 while longer segments appear in materials like nylon 612 and 1212.
These chain segments are referred to as aliphatic. Aliphatic structures tend to have greater molecular mobility than aromatic rings and are consequently often associated with lower-performing materials such as polyethylene and polypropylene. But in nylons these aliphatic segments still allow for a relatively high level of performance due to the strength provided by the amide groups.
The importance of the amide groups can be appreciated by observing how their spacing influences the properties of various nylons. Nylon 46 has a melting point (Tm) of 545 F/285 C while the melting point of nylon 612 is only 423 F/217 C. The glass-transition temperature (Tg) of most aliphatic nylons falls in the range of 140-167 F/60-75 C. When this same aliphatic structure was applied to polyester, the results were less impressive. The Tg was approximately room temperature and the Tm was below 392 F/200 C.
This difference illustrates the power of something called the hydrogen bond. When hydrogen is bonded to a very electronegative atom like nitrogen or oxygen, the sole electron surrounding the hydrogen nucleus is pulled away far enough to produce an unshielded positive charge. This becomes available to a neighboring molecule with a negative charge, such as the C=O bond in the nylon chain, forming a very strong attraction that requires a great deal of energy to break. This translates to strength, stiffness and heat resistance.
This hydrogen bond is the same feature that produces such a high boiling point in water. The ester group provides no such opportunity for hydrogen bonding, thus necessitating the use of aromatic rings in the polyester backbone to obtain a competitive property profile. Even with these rings, the Tg and Tm of the most common polyesters approaches or just matches those of the mainstream nylons.
But the presence of the hydrogen bond brings a cost. Because it is the same bond found in water, nylons are remarkably hygroscopic, absorbing 10 times as much water as polyesters. This produces the well-known change in mechanical and electrical properties that nylons undergo as they progress from the dry-as-molded to the conditioned state. Most data sheets for nylons list two sets of properties to document this change, and the strength and stiffness of an unfilled nylon at room temperature can be reduced by 50-60% while the volume resistivity may be reduced by four to five orders of magnitude.
Nylons are remarkably hygroscopic, absorbing 10 times as much water as polyesters.
In addition, parts fabricated from nylon undergo dimensional changes as they take on moisture. Polyesters do not exhibit these drastic changes as a function of exposure to the environment. When injection moldable polyesters were introduced to the market in the 1970s, their creators expected that this advantage would reduce the nylon market share significantly. The initial applications for polyesters were in the electrical industry, where the greater consistency of the material’s properties and molded dimensions made them preferred over nylon.
And yet, nylon remains a major factor for several reasons. First, the increased molecular mobility of the aliphatic structure in nylon allows for the achievement a higher degree of crystallinity. This provides superior property retention above Tg. Unfilled nylon 46 and 66 retain approximately 25% of their dry-as-molded room-temperature modulus above Tg while PBT retains only 15%. This differential becomes even greater when glass fibers are added. Of all the engineering resins commonly reinforced with glass fibers, nylons produce the greatest improvement in mechanical performance relative to the unfilled polymer. For example, adding 30% glass fiber to a PBT will double the tensile yield strength of the material, while in nylon 6 and 66 the improvement is 2.25-2.4 times that of the unfilled polymer.
These higher levels of crystallinity also provide for superior long-term mechanical performance. Creep and fatigue resistance are better in nylon 6 and 66 than they are in PBT and PET, despite the fact that moisture absorption significantly reduces these properties in nylon. In the late 1990s, one of my clients was experiencing problems with fatigue failures in 30% glass fiber-reinforced PET. After several unsuccessful attempts at reformulating the material, I suggested that they change to a glass-filled nylon 6. The PET resin supplier warned my client that while the nylon might perform better dry-as-molded, it would not work well once it had become conditioned. While moisture absorption did degrade the fatigue resistance, it was still better than that of the PET, and in the last 20 years not a single nylon part has been returned for fatigue failure.
In addition, in the 1980s, when commercial nylons were introduced that incorporated aromatic rings into the polymer backbone, the difference in performance between nylons and polyesters became that much greater. Today, partially aromatic nylons can achieve Tg’s as high as 284 F/140 C and melting points above 572 F/300 C while mitigating the effects of moisture absorption.
Both nylons and polyesters are susceptible to hydrolysis, the process of breaking covalent bonds in the polymer chain through a reaction with water. This can occur during processing if the materials are not properly dried, and is particularly problematic for PET. But it can also take place over a longer time period in molded parts if they are exposed to a combination of high heat and high humidity. Additives that provide resistance to hydrolysis have been commercially available for nylons for over 40 years, and improvements in this technology have produced grades that even allow for use of nylon 66 in automotive radiator parts. The technology for providing hydrolysis resistance in polyesters came along much later and focuses on PBT.
Additives that provide resistance to hydrolysis have been commercially available for nylons for over 40 years.
Despite these inherent strengths in the nylon family, polyesters have their place. The amide group is quite susceptible to oxidation, producing the well-known change in color that nylon exhibits when exposed to high temperatures. This mechanism also accounts for embrittlement during aggressive drying routines and the lower relative thermal index (RTI) values obtained with nylon, especially when retention of impact resistance is considered.
Even with the addition of heat-stabilizer additives, nylons struggle to obtain RTI values above 266 F/130 C, while PBT easily reaches 284 F/140 C and some grades of PET attain 311 F/155 C. Recent research into new heat-stabilization technologies in nylon have canceled some of this advantage, but most commercial nylon materials exhibit a lower level of resistance to oxidation than polyesters. UV resistance also tends to be better in polyesters.
A good example of the overlap in performance between these two families can be observed in the tire cord industry. Nylon was the first material to be adopted for tire cord and was initially the material of choice for the industry. But over time, polyester has made significant inroads and taken a significant share of the business. An objective comparison of the two material families as it relates to the properties that are important to the tire-cord industry still gives the nod to nylon, but polyester maintains a respectable share of the market.
In our next installment we will turn our historical attention to another semi-crystalline polymer that is frequently offered to the market by the same companies that provide nylons and polyesters. These are the acetal materials, otherwise known as polyoxymethylene (POM).
ABOUT THE AUTHOR: Michael Sepe is an independent materials and processing consultant based in Sedona, Ariz., with clients throughout North America, Europe, and Asia. He has more than 45 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 • mike@thematerialanalyst.com
Related Content
Tunnel Gates for Mold Designers, Part 1
Of all the gate types, tunnel gates are the most misunderstood. Here’s what you need to know to choose the best design for your application.
Read MoreHow to Get Rid of Bubbles in Injection Molding
First find out if they are the result of trapped gas or a vacuum void. Then follow these steps to get rid of them.
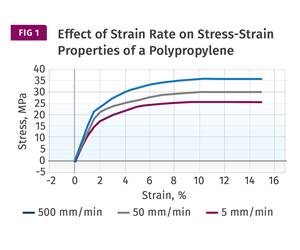
Read MoreUnderstanding Strain-Rate Sensitivity In Polymers
Material behavior is fundamentally determined by the equivalence of time and temperature. But that principle tends to be lost on processors and designers. Here’s some guidance.
Read MoreThe Importance of Melt & Mold Temperature
Molders should realize how significantly process conditions can influence the final properties of the part.
Read MoreRead Next
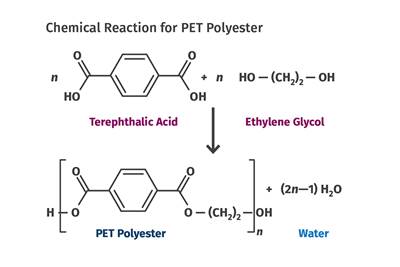
Tracing the History of Polymeric Materials: PET
How PET evolved from a material for fibers and fabrics to a force in packaging.
Read MoreTracing the History of Polymeric Materials: Polyesters
Beyond PET, PBT and their analogues, development of polyester chemistry led to unsaturated thermosetting resins, copolyester thermoplastic elastomers, liquid-crystal polymers and, most recently, biopolymers.
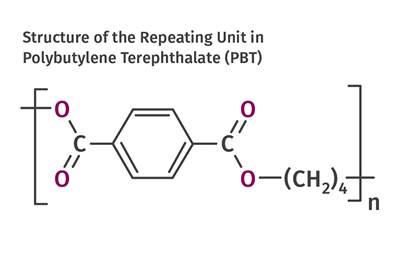
Read MoreTracing the History of Polymeric Materials: PBT
The slow crystallization of PET polyester made it a poor option for processes like injection molding. This led to the development of more molder-friendly options such as PBT.
Read More
.jpg;width=70;height=70;mode=crop)


















 (2).jpg;maxWidth=300;quality=90)