PBT and PET Polyester: The Difference Crystallinity Makes
To properly understand the differences in performance between PET and PBT we need to compare apples to apples—the semi-crystalline forms of each polymer.
We have previously (September 2012) profiled similarities and differences between different chemistries in the acetal polymer family. This month we will begin making a similar comparison between different commercially available polyesters known as PBT and PET. Many years ago, while working for a custom injection molder, I worked closely with one of our customers to convert a series of parts from 30% glass-fiber reinforced PBT to a PET with the same level of filler. It served as a practical application of the differences between the materials on both processing and performance levels.
Fundamentally, the chemistry of PET and PBT is very similar. Polyesters are synthesized by reacting an organic acid, in this case terephthalic acid, with an alcohol. In the case of PBT the alcohol is generically referred to as butylene glycol while in PET it is ethylene glycol. The resulting polymers are known, therefore, as polybutylene terephthalate (PBT) and polyethylene terephthalate (PET).
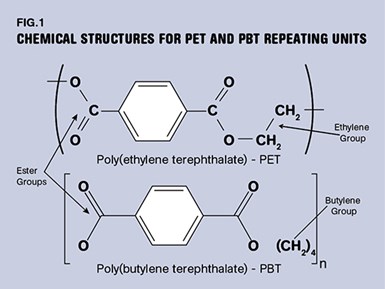
The differences in the materials are best understood by examining the chemical structure of the repeating unit that makes up the polymer chains, as shown in Fig. 1. The essential feature that makes the materials distinctive is the ester group that lends its name to this family of materials. Other polymers such as PTT and PCT are also members of this chemical family that display slight variations on these structures.
Another key feature of the chemistry in this material is the six-sided ring that appears at regular intervals in the backbone. Known as a phenyl ring or more generally as an aromatic ring, this element provides stiffness to the polymer chain. This influences several important properties, including the glass-transition temperature, a region where polymers lose a significant percentage of their load-bearing properties.
Fundamentally, the chemistry of PET and PBT is very similar.
It is not evident from a two-dimensional rendering of the chemical structure, but a 3D view would show that while many of the chemical groups in a polymer chain project into or out of the page, the aromatic ring sits in a plane. It also constrains the natural tendency of the other groups in the chain to rotate and vibrate. This is part of the stiffening effect of this ring structure. The reduced mobility and the bulky nature of the ring also influence the ability of the polymer to crystallize as it cools. PBT, with greater spacing between the aromatic rings, crystallizes more efficiently than PET. But PET, if it is successfully crystallized, provides for better mechanical properties, including strength, stiffness, and performance at elevated temperature.
Most consumers are familiar with PET in the containers that holds their bottled water or soft drinks. This type of PET is amorphous and is engineered to prevent crystallization. If bottle-grade PET did crystallize it would become cloudy and, more importantly, it would lose its impact resistance. So while there are probably a lot of parts molded in crystalline PET polyester under the hood of your car, where they encounter elevated temperatures and aggressive chemical environments; the vast majority of the PET in the world is consumed in packaging, where it is amorphous, unreinforced, and incapable of handling such rigorous environments.
The type of PET that we will be discussing in Part 2 of this article is semi-crystalline and almost always contains high levels of glass fibers and/or mineral fillers. PBT polyester, however, can be provided in its semi-crystalline form both filled and unfilled. In fact, because PBT crystallizes faster than PET, it is not possible under normal processing conditions to produce PBT parts that are amorphous. The polymer crystallizes efficiently enough to always achieve some level of organization in its structure. The stiffness of the ester groups and the aromatic rings is balanced by the flexibility and mobility of the butylene group. But in PET the shorter ethylene group makes crystallinity optional. We can have amorphous PET if we cool it rapidly or we can have semi-crystalline PET if we cool it slowly.
Most PET bottles start out as injection molded preforms. They are clear and tough and relatively thick-walled in order to allow for the thinning effect that the wall will undergo when the preform is reheated and stretched to form the bottle. If you have worked in a bottle manufacturing plant you know that if, during the preheat cycle, the preforms get too hot they turn cloudy—a sign of crystallization. (In fact, if you look closely at the gate area of a preform you will see a small amount of haze in this area due to the extra heat generated in this area of the part).
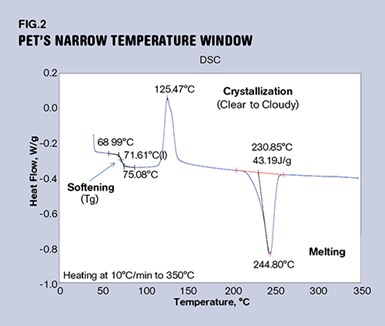
If you try to blow a bottle from this cloudy, partly crystallized material, it will have reduced impact performance. The preform may even shatter during the blowing process if enough crystallinity develops. So the trick is to keep the material above its glass-transition temperature, but below its crystallization temperature. This temperature window may not be very wide, as can be seen in Fig. 2.
This graph shows the behavior of amorphous PET polyester, an unfilled clear material that is used to make parts that require toughness and transparency but do not need to withstand elevated temperatures. As the material is heated from room temperature, the first notable event is the glass transition. This appears as a step change in the heat content of the material, and in this compound that process is complete at 75 C (167 F). At this point the material has lost the rigidity it possessed at room temperature and is soft and pliable. As the temperature is increased, the viscosity of the softened polymer will decrease until it reaches a temperature near 110 C (230 F). This is the temperature at which the baseline of the scan starts to rise rapidly, and the gap between 75 C and 110 C represents the window of opportunity for blowing the bottle.
Most PET bottle plants that I have visited are running a preheat temperature near 100 C (212 F). Once the crystallization process has started, the material begins to turn cloudy. It will also begin to regain some of the stiffness that it lost as it went through the glass transition. If this process goes far enough, the polymer becomes crystallized at about 140 C (284 F). At this point the material will be opaque and brittle and will stay that way until the crystal structure melts at about 245 C (473 F). So PET can go either way—amorphous or semi-crystalline—depending on how we treat it.
But PBT is always semi-crystalline under normal commercial circumstances. So to properly understand the differences in performance between PET and PBT we need to compare apples to apples—the semi-crystalline forms of each polymer. Because PET crystallizes very slowly, producing parts with a semi-crystalline structure requires the help of chemicals known as nucleating agents, as well as the presence of solid particles of fillers and reinforcements. Thus commercial semi-crystalline PET polyesters are always sold filled or reinforced, and to make a fair comparison of PET vs. PBT performance we therefore need to compare the materials with an equivalent level of the same type of filler. We will do this in Part 2 of this investigation and also discuss the differences in processing that molders will encounter as they work with these two polymer families.
ABOUT THE AUTHOR: Michael Sepe is an independent materials and processing consultant based in Sedona, Ariz., with clients throughout North America, Europe, and Asia. He has more than 45 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 • mike@thematerialanalyst.com
Related Content
The Fantasy and Reality of Raw Material Shelf Life: Part 1
Is a two-year-old hygroscopic resin kept in its original packaging still useful? Let’s try to answer that question and clear up some misconceptions.
Read MoreTracing the History of Polymeric Materials: Polyphenylene Oxide
Behind the scenes of the discovery of PPO.
Read MoreMelt Flow Rate Testing–Part 1
Though often criticized, MFR is a very good gauge of the relative average molecular weight of the polymer. Since molecular weight (MW) is the driving force behind performance in polymers, it turns out to be a very useful number.
Read MoreScaling Up Sustainable Solutions for Fiber Reinforced Composite Materials
Oak Ridge National Laboratory's Sustainable Manufacturing Technologies Group helps industrial partners tackle the sustainability challenges presented by fiber-reinforced composite materials.
Read MoreRead Next
How Do You Like Your Acetal: Homopolymer or Copolymer?
Acetal materials have been a commercial option for more than 50 years.
Read MoreHow Polymer Melts in Single-Screw Extruders
Understanding how polymer melts in a single-screw extruder could help you optimize your screw design to eliminate defect-causing solid polymer fragments.
Read More.jpg;width=70;height=70;mode=crop)