Hygroscopic vs. Non-Hygroscopic Resins
What does hygroscopic mean, and how does it affect resin moisture retention and drying?
Hygroscopic Resins
(i.e., Nylon, ABS, Acrylic, Polyurethane, Polycarbonate, PET, PBT,)
- Have a strong affinity to attract moisture
- Will absorb moisture onto their molecular structure if exposed to ambient air
- Internal moisture can not be removed with hot air alone
Water vapor surrounding a hygroscopic pellet is absorbed into the pellet. As the vapor pressure within the pellet increases to equal the vapor pressure surrounding the pellet, equilibrium occurs. This is referred to as, moisture equilibrium.
When an environment of hot, dry air surrounds a wet hygroscopic pellet, the vapor pressure surrounding the pellet is lower than the vapor pressure within the pellet. Consequently, the moisture within the pellet begins to migrate toward the area of low vapor pressure outside the pellet. Expose the pellet to the hot, dry atmosphere for a sufficient period and the pellet eventually reaches moisture equilibrium with the surrounding dry conditions. In other words, the pellet becomes dry.
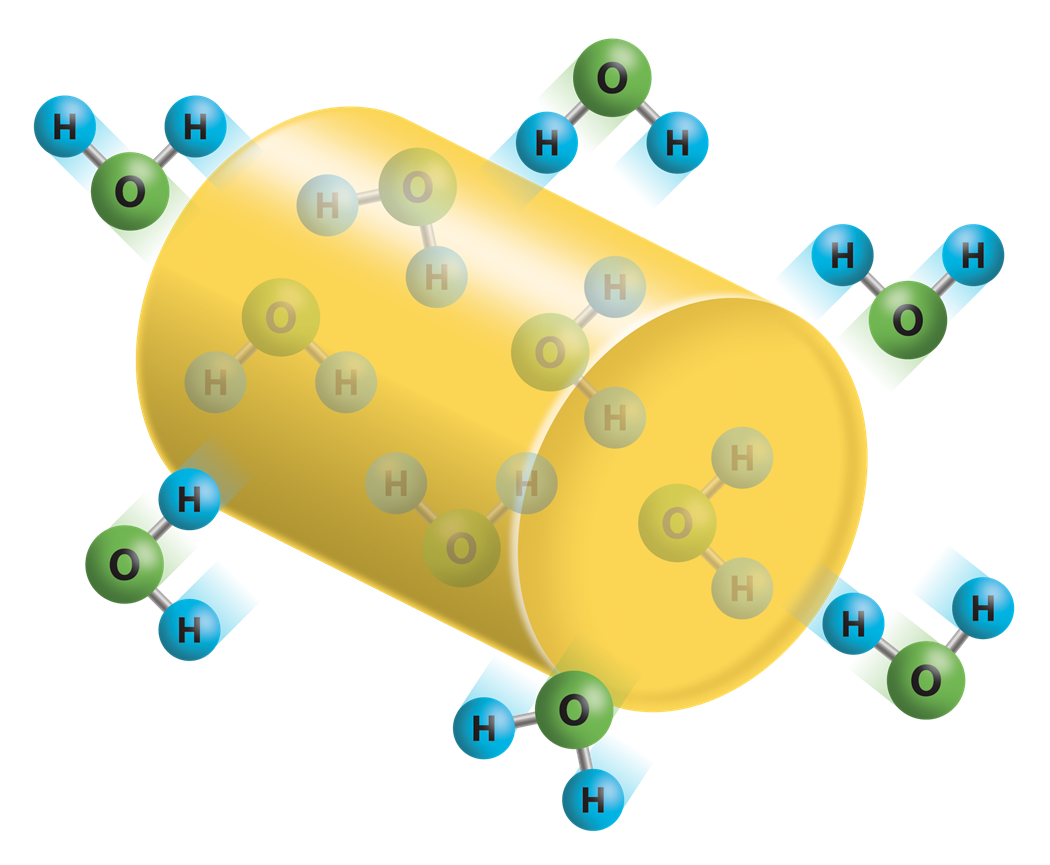
Hygroscopic plastic pellets have a strong affinity to attract moisture and absorb it into the molecular structure of the pellet. Photo Credit: Novatec
Non-Hygroscopic Resins
(i.e., Polyethylene, Polypropylene, Polystyrene, PVC)
- Do not have an affinity for moisture
- Any moisture collected is adsorbed on the surface of the pellet
- Typical moisture collection is due to condensation
- Moisture is easily removed by passing a sufficient stream of warm air over the material
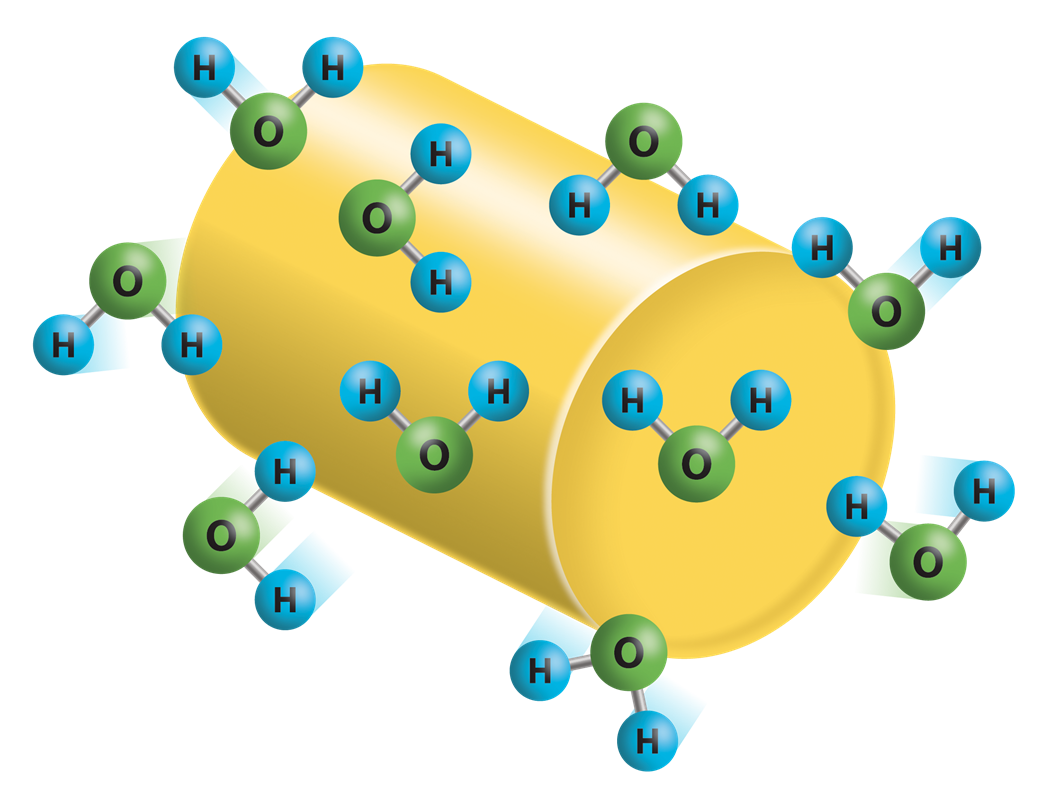
Non-hygroscopic polymers do not absorb moisture from the atmosphere into the pellet but may have moisture on the pellet's surface. Photo Credit: Novatec






