Materials Part 1: What Annealing Can Do for Your Process
Relatively rapid cooling rates in processing introduce internal stress. If functional problems in use result, annealing may draw down the stress to levels that may not be achievable during processing.
Long before there were plastics there was the process of annealing. The metals industry, and more specifically the steel industry, has known for a long time that conducting a follow-up process that exposes materials to controlled heating and cooling will reduce the hardness of the material, increase ductility, and reduce internal stresses. The microstructure of the material is also changed. Other metallic materials such as copper and brass can also benefit.

Annealing can relieve stresses in amorphous materials and increase crystallinity in semi-crystalline resins. (Photo: Annealing oven from Grieve Corp.)
Annealing of plastics is not performed as part of most manufacturing processes. There are exceptions. Products of significant thickness such as solid rod, thick-walled tubing and sheet are often annealed as a preparatory step for machining. This is done to stabilize the structure of the material and reduce internal stress, much the same reasons that the process is conducted in metallic materials. In all products fabricated by melt processing, the relatively rapid cooling rates that are associated with these processes introduce some level of internal stress and a departure from an equilibrium state. In cases where this produces a level of internal stress that creates functional problems in use, annealing may be performed to draw down the stress to levels that may not be achievable during processing.
Products of significant thickness such as solid rod, thick-walled tubing and sheet are often annealed as a preparatory step in machining.
The rationale for annealing, and the effect that it has on the material, will depend greatly upon the polymer being annealed. In amorphous polymers the objective is to reduce internal stress. Parts that are produced in a well-controlled process that gives appropriate attention to the importance of cooling rate may contain internal stresses below 1000 psi. But parts that are rapidly cooled may display internal stresses two to three times higher. The higher the internal stress is, the less capable the product will be of managing external stresses without failing. In addition, failures in parts that contain a high level of internal stress are more likely to be brittle.
Even if the application is not expected to involve an elevated level of external stress, high internal stresses can increase susceptibility to environmental stress cracking (ESC). Amorphous polymers are particularly likely to exhibit ESC if they are exposed to certain chemical agents. These chemical agents may be present as solvents, plasticizers, cleaning agents, rust preventatives and adhesives, and prolonged contact of an amorphous polymer with these fluids can result in ESC failures. In these types of environments annealing can be the difference between success and failure.
In semi-crystalline polymers the purpose for annealing is fundamentally different. Semi-crystalline polymers are used because of the mechanical and thermal attributes that arise from their crystallinity. The degree of crystallinity governs properties such as strength, modulus, retention of mechanical properties above the glass-transition temperature, chemical resistance, fatigue and creep resistance, and tribological properties. Just as internal stresses in amorphous polymers are minimized by slower cooling rates, crystallinity in a semi-crystalline polymer is maximized by slowing the rate at which the material is cooled.
But even in the best of circumstances, the cooling rates associated with melt processing result in a part that possesses approximately 90% of the achievable crystallinity. In most cases, this is sufficient. But in those instances where it is not, annealing is performed to provide that additional 10%.
The opportunity for crystal formation occurs in a temperature window below the melting point of the polymer and above its glass-transition temperature (Tg). Consequently, the annealing temperature must be above the Tg in order to achieve the desired result. Optimal crystallization rates are usually obtained near the midpoint between the melting point and the Tg. As an example, nylon 66, with a Tg of 60 C (140 F) and a melting point of 260 C (500 F), anneals most efficiently at around 160 C (320 F).
In crosslinked materials, the annealing process is performed for reasons similar to those that govern semi-crystalline thermoplastics. Just as molding processes struggle to achieve the highest level of crystallization possible, they also do not typically achieve the optimal level of crosslinking. While this may be accomplished by extending the cycle time, the economics often do not favor such an approach and it is more efficient to reheat a large number of parts after molding. In the thermoset industry this is typically referred to as post-baking and it is most often performed on polymers such as phenolics and polyimides.
However, many practitioners in the industry have also found benefits in performing this operation on unsaturated polyesters, epoxies, and silicones. In order for the post-baking process to effectively advance the crosslink density of the material, the temperature of the baking process must exceed the Tg of the polymer in the molded part. As we will see in a later article, there are some thermoplastics that also require post-baking in order to achieve optimal properties.
Some elastomers also benefit from a post-baking or annealing process. As with semi-crystalline thermoplastics and rigid crosslinked polymers, the objective is not reduction of internal stress, but instead a structural rearrangement that improves mechanical and thermal performance. This process can be useful in thermoplastic elastomers such as polyurethanes, and it has also been shown to improve performance in crosslinked systems such as silicone rubber. The process is particularly useful in providing optimal performance in applications where prolonged exposure to elevated temperatures are involved.
In order for certain processes to achieve the desired result, the specific conditions of annealing or post-baking temperature and time are critical.
In order for these processes to achieve the desired result, the specific conditions of annealing or post-baking temperature and time are critical. Of equal importance in some of these cases is the rate of cooling after the heating process is concluded. Failure to manage this cooling process is often the reason that annealing does not achieve the desired result. This is a parameter that is often overlooked.
In subsequent articles in this series, we will discuss the different requirements that pertain to amorphous thermoplastics, semi-crystalline thermoplastics, crosslinked materials, and elastomers. We will also discuss the limits of this process to achieve positive outcomes without introducing unintended negative consequences.
ABOUT THE AUTHOR: Mike Sepe is an independent, global materials and processing consultant whose company, Michael P. Sepe, LLC, is based in Sedona, Ariz. He has more than 40 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 • mike@thematerialanalyst.com.
Related Content
How Much L/D Do You Really Need?
Just like selecting the extruder size and drive combination, the L/D should be carefully evaluated.
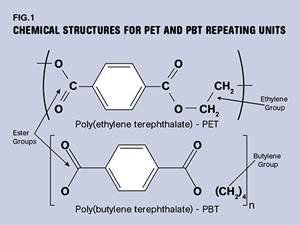
Read MorePBT and PET Polyester: The Difference Crystallinity Makes
To properly understand the differences in performance between PET and PBT we need to compare apples to apples—the semi-crystalline forms of each polymer.
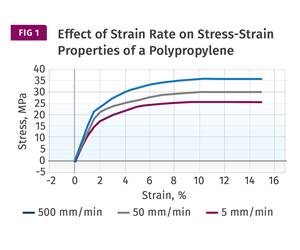
Read MoreUnderstanding Strain-Rate Sensitivity In Polymers
Material behavior is fundamentally determined by the equivalence of time and temperature. But that principle tends to be lost on processors and designers. Here’s some guidance.
Read MoreAre Your Sprue or Parts Sticking? Here Are Some Solutions
When a sprue or part sticks, the result of trying to unstick it is often more scratches or undercuts, making the problem worse and the fix more costly. Here’s how to set up a proper procedure for this sticky wicket.
Read MoreRead Next
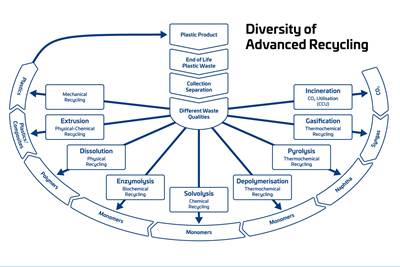
Advanced Recycling: Beyond Pyrolysis
Consumer-product brand owners increasingly see advanced chemical recycling as a necessary complement to mechanical recycling if they are to meet ambitious goals for a circular economy in the next decade. Dozens of technology providers are developing new technologies to overcome the limitations of existing pyrolysis methods and to commercialize various alternative approaches to chemical recycling of plastics.
Read MoreTroubleshooting Screw and Barrel Wear in Extrusion
Extruder screws and barrels will wear over time. If you are seeing a reduction in specific rate and higher discharge temperatures, wear is the likely culprit.
Read MoreLead the Conversation, Change the Conversation
Coverage of single-use plastics can be both misleading and demoralizing. Here are 10 tips for changing the perception of the plastics industry at your company and in your community.
Read More
.jpg;width=70;height=70;mode=crop)





.png;maxWidth=300;quality=90)