Tracing the History of Polymeric Materials: Polycarbonate
How polycarbonate came about, virtually simultaneously, through the efforts of two industry giants.
Throughout the 1930s the development and commercialization of new polymers occurred at a rapid pace. PVC, polystyrene, polyethylene, acrylic, and moldable cellulosics all came of age in this decade. By the 1940s, the properties of polystyrene had been extended by adding butadiene rubber to improve the impact of the general-purpose material at the expense of its transparency. The properties of polystyrene were also extended by copolymerizing with acrylonitrile to make SAN, a clear material with improved heat and chemical resistance. This material was also toughened by the addition of butadiene rubber to make ABS, again with transparency as a casualty.

Polycarbonate has become a go-to material wherever transparency , heat resistance and toughness are required. (Photo: PolyOne)
Today we consider the early transparent polymers as generally either hard and brittle, such as SAN and acrylic, or soft and tough, such as the cellulosics. But during this period of early development, many of these materials were offered as a safe alternative to glass. This was particularly the case for acrylics, developed largely through the efforts of chemists like Otto Rohm. Acrylics remain a very versatile alternate to glass, largely due to their outstanding resistance to the effects of weathering and ultraviolet radiation. But the compromise between transparent materials that were soft and those that were hard and brittle changed dramatically in the early 1950s with the creation of a completely new chemistry that we know today as polycarbonate.
The development of PC shares common themes with many of the materials we have discussed previously. Its development occurred over an extended period of time with multiple false starts. The discovery of the material that was ultimately successful commercially was accidental, and it occurred nearly simultaneously in different parts of the world. While we associate the beginning of PC production with the late 1950s, the material was first created in the laboratory in 1898 by the German chemist Alfred Einhorn, who worked and taught at the University of Munich with Adolf von Baeyer, the Nobel Prize winning chemist who first synthesized and then abandoned phenolic.
Einhorn is best known as the person who was the first to synthesize the local anesthetic procaine, which became better known as Novocain. But in the 1890s, Einhorn was attempting to synthesize cyclic carbonates and produced polycarbonate by reacting hydroquinone with phosgene. Thirty years of experimentation in the lab by various chemists produced no commercial success.
But the chemical reaction was the prototype of the one that eventually produced the polymer we use today. In 1928, Wallace Carothers and his team at DuPont also produced PCs while working on the development of polyesters and nylons. Chemically, PC and polyester possess a lot of similarities, and the condensation reactions that Carothers was employing to produce his materials were very similar to those used ultimately to polymerize polycarbonate.
While we associate the beginning of PC production with the late 1950s, the material was first created in the laboratory in 1898.
But the materials that Carothers produced had an aliphatic backbone structure as opposed to the aromatic backbone that characterized the commercial product. Consequently, the thermal, mechanical, and chemical properties of these early polycarbonates were not considered to be useful and these materials were also shelved.
It was not until 1953 that Hermann Schnell and his team, working at the Bayer facility in Uerdingen, Germany, created the version of polycarbonate that became the platform for its current commercial success. One week later, Dan Fox at General Electric independently stumbled upon the same compound while working to develop a new wire-insulating material. The two versions of the polymer were chemically the same but differed structurally. The Bayer material, trade named Makrolon in 1955, was a linear polymer, while the GE discovery was a branched material.
The properties of the two materials were very similar. They were transparent, although the initial versions had an amber tint that was only resolved in 1971 when chemical impurities were eliminated. The materials also had a softening point that was approximately 50º C (90º F) higher than any of the known transparent materials, comfortably above the boiling point of water. And they possessed a level of ductility that far exceeded any other transparent material known at the time. In other words, polycarbonate extended the range of applications that could be fulfilled by clear polymers beyond anything that had been previously possible.
PCs created by GE and Bayer were chemically the same but differed structurally.
The nearly simultaneous development of the polymer by two large companies could be expected to produce a significant level of legal activity. But unlike the 30-year saga of legal action that characterized the development of HDPE and PP, the potential battle over the rights to PC was settled relatively quickly with an agreement that the firm that was ultimately given priority would grant a license to the other company. The priority was given to Bayer, although if you were to read the sales literature coming out of General Electric in the 1970s and 1980s or if you were to visit Pittsfield, Mass., during that time, you could be forgiven for concluding that all of the intellectual property associated with PC resided within the halls of GE.
In 1978 I visited Pittsfield during the Christmas holiday. At that time GE employed over 5000 people in a town with a population of just over 50,000. These employees worked at the Power Transformer Division, the Naval Ordinance Division, or in the Plastics Division. As we drove by the GE facilities, I noticed that the holiday decorations in front of the first two complexes were decidedly understated while the front of the Plastics Division building looked like Chevy Chase’s house in the film “Christmas Vacation.” The person I was traveling with commented that it was easy to tell which division was making all the profit.
Today neither Bayer or GE own the rights to this breakthrough material. In 2006 GE sold its entire Plastics Division to Saudi Arabia Basic Industries (SABIC). In 2015 the Material Science Division of Bayer spun off its interest in polymers to Covestro while under the direction of a leader who had performed brilliant research in polymer blends for General Electric in the 1980s and early 1990s. It is a commentary on how the plastics industry has changed from a fast-track growth business to a more mature endeavor over the last 20 years, and how the focus of large corporations on capital investment has shifted away from the world of polymers.
Nevertheless, the years after the discovery were heady times. Production of polycarbonate began at Bayer in 1958 and at General Electric in 1960. Applications developed rapidly in the electrical and electronics fields, construction and lighting, food packaging, the automotive and aerospace industries, and medical devices. In any application where clarity and toughness were required, PC became the material of choice. This included eyewear, windscreens, fairings and safety shields for motorcycles and ATVs, and even cockpit canopies for aircraft. The polymer proved to be extremely versatile, manufacturable in the form of film, where it found use as a very effective electrical insulator, but also lending itself to blow molding, extrusion, injection molding, and thermoforming.
In any application where clarity and toughness were required, PC became the material of choice.
In 1981 the material entered the data storage market with the creation of the first compact disks, followed soon after by DVDs. The high glass-transition temperature of the material made it suitable for sterilization by steam and autoclave techniques, and the aromatic chemistry of the polymer backbone coupled with the amorphous structure has also made it a leading candidate for high-energy sterilization techniques such as gamma and E-beam. Grades have been developed that meet standards for biocompatibility as well as food contact. UV-stabilized grades were developed to extend the utility of the material into outdoor applications. The first flame-retardant grades were developed in 1970.
Polycarbonate also has been one of the most often utilized polymers in blends. These improve some of the properties in areas where the material is considered to be vulnerable. Blends have been created with ABS, SAN, ASA, polyesters, and polyurethanes. Global production capacity reached 10 billion lb in 2016. Copolymers with polyesters have extended the operating temperatures close to 200 C (392 F) and the incorporation of siloxane chemistry into the polymer backbone has improved resistance to hydrolysis and environmental stress-cracking, two influences that have always been notable weaknesses in the performance profile of the material.
Despite all this success, the history of PC has not been without its challenges. We will take a look at some of those in our next installment.
ABOUT THE AUTHOR: Michael Sepe is an independent materials and processing consultant based in Sedona, Ariz., with clients throughout North America, Europe, and Asia. He has more than 45 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 •mike@thematerialanalyst.com
Related Content
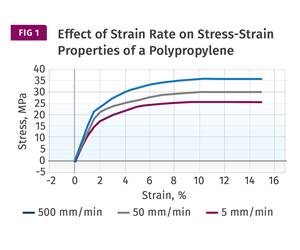
The Strain Rate Effect
The rate of loading for a plastic material is a key component of how we perceive its performance.
Read MoreHow Much L/D Do You Really Need?
Just like selecting the extruder size and drive combination, the L/D should be carefully evaluated.
Read MoreDensity & Molecular Weight in Polyethylene
This so-called 'commodity' material is actually quite complex, making selecting the right type a challenge.
Read MoreUnderstanding Strain-Rate Sensitivity In Polymers
Material behavior is fundamentally determined by the equivalence of time and temperature. But that principle tends to be lost on processors and designers. Here’s some guidance.
Read MoreRead Next
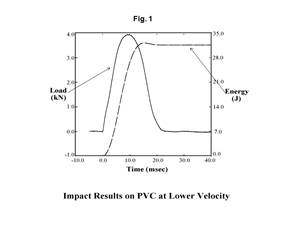
Tracing the History of Polymeric Materials: PVC & PVDC
How a class of materials based on chlorine chemistry became part of the landscape.
Read MoreTracing the History of Polymeric Materials: Nylon
The story of nylon, the first true engineering thermoplastic.
Read MoreTracing the History of Polymeric Materials: Teflon
The accidental discovery of Teflon.
Read More.jpg;width=70;height=70;mode=crop)