Tracing the History of Polymeric Materials: How Nylons & Polyesters Connected
The history of nylons and polyesters are intertwined, and it takes some knowledge of chemistry to understand why.
The same wave of innovation that produced the development of nylon also created synthetic polyesters. Julian Hill, a member of the team at DuPont led by Wallace Carothers, first synthesized polyesters that could be spun into fibers. This occurred before the development of nylon; however, once the properties of nylon became apparent, the work on polyesters was set aside. The history of nylons and polyesters have been intertwined ever since, and to appreciate the reason for this it is useful to understand a little chemistry.
Both polyesters and the first nylons are condensation polymers. The reactions that are used to create these materials were beginning to be considered by Carothers as early as 1926, when he was still in the academic world. Once he had the resources of the DuPont labs at his disposal, he quickly moved to turn theory into practice. Condensation polymers are created by reacting certain types of chemicals that have functional groups on both ends of the molecule, so that the reaction can extend the resulting product in both directions, thus producing a long chain.
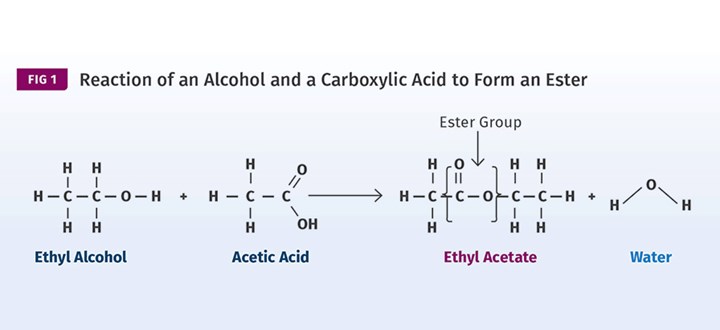
Esters are made by reacting alcohols with carboxylic acids. Shown here, ethyl alcohol is combined with acetic acid to produce the ester, ethyl acetate. (Images: Mike Sepe)
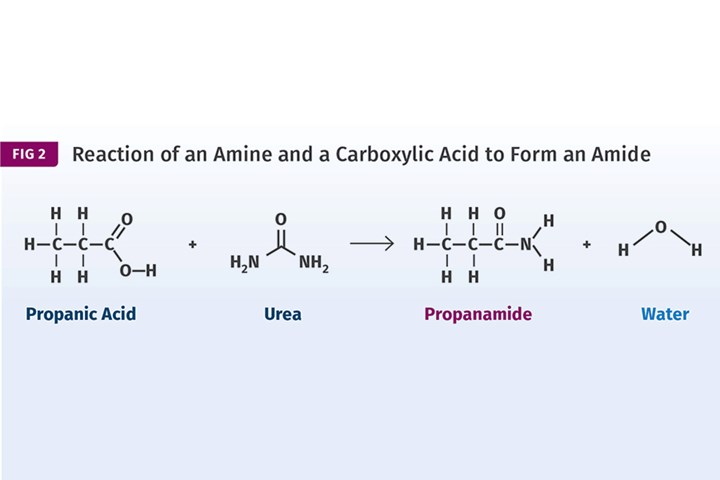
Esters are made by reacting alcohols with carboxylic acids. An example is shown in Fig. 1 where ethyl alcohol is combined with acetic acid to produce the ester, ethyl acetate. (In organic chemistry, if the name of the compound ends in “-ate,” it is almost certainly an ester.) The ester contains the distinctive group highlighted in the image. Amides are made in a similar manner, except that instead of an alcohol we use an amine. This is shown in Fig. 2. In this reaction, propanoic acid is combined with the amine urea to produce propanamide.
In both of these cases, one or both of the reactants have a reactive group on only one end of the molecule, so once the reaction takes place the process is over. However, Carothers and his team discovered that if they used reactants with functional groups on both ends of the molecule, the reaction could be extended to create a long-chain macromolecule, a polymer. Figure 3 shows this principle applied to nylon 66. Polyester, which had actually been synthesized earlier using the same type of reaction, was put on the shelf while all the focus shifted to nylon.
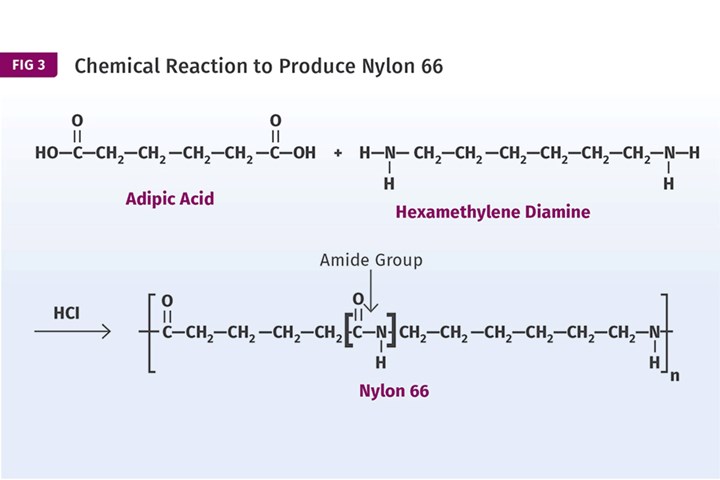
If reactants with functional groups are used on both ends of the molecule, the reaction could be extended to create a long-chain macromolecule—a polymer. This principle is applied here to nylon 66.
To understand the reason for this, we need to appreciate the chemistry of the ester and the amide groups. The amide group consists of a nitrogen-carbon bond within the polymer backbone. Attached to the carbon by a double bond is an oxygen atom, while a hydrogen atom is attached to the nitrogen. This sets up a very fortuitous situation. Both of these segments of the amide group behave as little magnets with a well-defined positive and negative pole. In the case of the C=O bond, the oxygen is negatively charged, while in the N-H bond the hydrogen is positively charged. When neighboring segments of polymer chains align, the attractive forces between the negatively charged oxygen and the positively charged hydrogen are very strong and result in superior mechanical properties and a very high melting point.
It was the melting point of 260 C (500 F), as well as the very high strength and modulus of the material, that drew the attention of the DuPont researchers. The presence of the hydrogen bonded to the nitrogen is key here. Hydrogen is the simplest element in our universe, consisting of a nucleus that contains a single positively charged proton around which a single negatively charged electron orbits. When any atom enters into a chemical reaction, it does so by sharing at least one electron with a partner.
In the case of hydrogen, sharing this electron leaves the positive nucleus unshielded. If the partner atom is able to remove the hydrogen electron far enough from its nucleus, the positive nucleus becomes capable of participating in a very strong attractive force known as a hydrogen bond. Only three elements are able to pull the hydrogen electron far enough from its nucleus to create such a hydrogen bond and nitrogen is one of them. When these strong positive charges align with the negatively charged oxygen atoms, as shown on Fig. 4, the forces binding these segments become very strong and the excellent properties of nylon are the result.
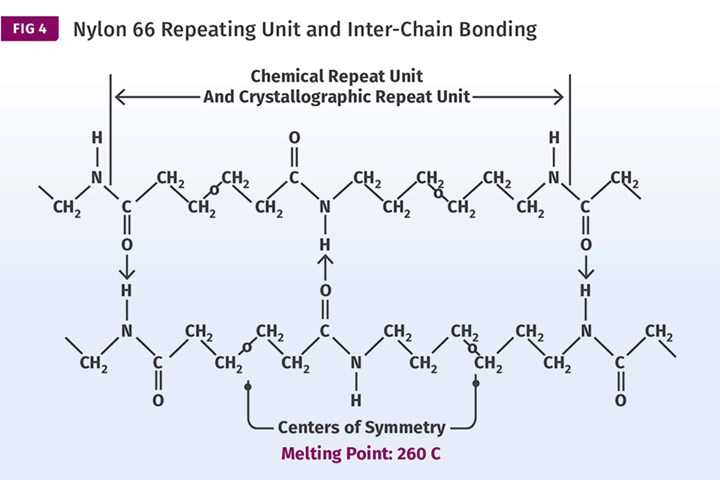
It is important to note that the structure of the nylon polymer between the amide groups consists only of a chain of carbon with hydrogen atoms attached to the side of the chain. This resembles the chemical structure of polyethylene, a simple molecular geometry that is referred to in chemistry as an aliphatic structure. Aliphatic structures do not usually result in very impressive thermal and mechanical properties. But it works in polyamides because the combination of the strong attractions provided by the hydrogen bonds, coupled with the symmetry of the amide group spacing, provides properties that are well beyond what would typically be expected.
Aromatic rings are an indispensable part of modern polymer chemistry, but in the 1930s their use was still limited to thermosetting polymers such as phenolic.
So why did polyesters get put on the back burner in favor of the nylon chemistry? If we return to the structure in Fig. 1, we can see that the ester group, while similar to that of the amide group, is missing the opportunity for forming hydrogen bonds. In place of the N-H group we have only an oxygen atom. The absence of the hydrogen bonding resulted in aliphatic polyesters with much lower melting points and much lower strength. An aliphatic polyester with an average molecular weight comparable to that of nylon 66 would have a melting point of only 80 C (176 F) and would also exhibit a significant tendency to undergo hydrolysis. These properties did not fulfill the expectations of the DuPont researchers, who were focused primarily on fibers for fabric and clothing.
Therefore, while the creation of polyester and its formation into fibers in the lab preceded nylon by about three years, nylon received all the emphasis in development and polyester was placed on the shelf. In the latter part of the 1930’s, two British researchers, John Rex Whinfield and James Tennant Dickson, began reviewing the work that the Carothers team at DuPont had performed on polyesters. They discovered that the DuPont team had not pursued the avenue of improving the property profile of polyester by using chemistry that replaced the original aliphatic structures with a type of chemistry known as aromatic. In chemistry the term aromatic has a very particular meaning: It refers to a ring-like chemical structure that typically involves six carbons and is exemplified by substances such as benzene and xylene.
Aromatic rings are an indispensable part of modern polymer chemistry, but in the 1930s their use was still limited to thermosetting polymers such as phenolic. These rings are planar and very rigid and therefore they impart significant improvements in thermal and mechanical performance when they are incorporated into organic compounds and polymers. In 1939, Whinfield and Dickson used an aromatic carboxylic acid, terephthalic acid, in combination with ethylene glycol, to produce the first commercially viable polyester, polyethylene terephthalate (PET). Working with two British inventors, W.K. Birtwhistle and C.G. Ritchie, they patented PET polyester and commercialized a fiber based on the material that was introduced by Imperial Chemical Industries (ICI) in 1941 as Terylene.
This development started the long history of polyesters that we will chronicle in our next installment.
ABOUT THE AUTHOR: Michael Sepe is an independent materials and processing consultant based in Sedona, Ariz., with clients throughout North America, Europe, and Asia. He has more than 45 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 • mike@thematerialanalyst.com
Related Content
Let's Take a Journey into the World of Molding Thermosets – Part 1
There are many fundamental differences between thermosets and thermoplastics, from the way raw materials are furnished to the molder and the process in which parts are molded.
Read MorePrices of All Five Commodity Plastics On the Way Up
Despite earlier anticipated rollover in prices for most of the volume commodity resins, prices were generally on the way up for all going into the third month of first quarter.
Read MorePolymer Science for Those Who Work With Plastics: Molecular Weight — What It Is and Why It Matters
Molecular weight might seem like an abstract concept, but it plays a crucial role in determining the behavior of plastics during processing and in their final applications.
Read MorePolymer Science for Those Who Work With Plastic — Part 1: The Repeat Unit
What are the basic building blocks of plastics and how do they affect the processing of that material and its potential applications in the real world? Meet the repeat unit.
Read MoreRead Next
Tracing the History of Polymeric Materials: Teflon
The accidental discovery of Teflon.
Read MoreTracing the History of Polymeric Materials: More on Polycarbonate
The industry has learned a lot about the advantages and disadvantages of polycarbonate during its more than 60-year history, and it would be difficult to imagine a world in which it did not exist.
Read MoreTracing the History of Polymeric Materials: Polycarbonate
How polycarbonate came about, virtually simultaneously, through the efforts of two industry giants.
Read More
.jpg;width=70;height=70;mode=crop)




















