Tracing the History of Polymeric Materials: More on Polycarbonate
The industry has learned a lot about the advantages and disadvantages of polycarbonate during its more than 60-year history, and it would be difficult to imagine a world in which it did not exist.
When polycarbonate was first created it appeared to be a material that was all upside. Transparent, impact resistant, and able to withstand the short-term effects of exposure to boiling-water temperatures, PC extended the performance range traditionally associated with clear materials.
But in the late 1990s I was attending a presentation at the Society of Plastics Engineers’ ANTEC on failure analysis, and the presenter made a surprising statement: Based on a meta-analysis of almost 5000 case studies in which his company had participated, they observed that while by weight PC accounted for 1% of all the plastic consumed annually, about 15% of their failure studies involved a part made from PC.
Weight of the failed product is probably not the best metric to use, given the wide range of sizes and masses associated with plastic parts. Nevertheless, such a ratio deserves some attention. As someone who has conducted about 6000 failure-analysis studies, I can attest to the disproportionately large number of times that PC is the material involved in the failure. And most of these failures present as brittle; not what would be expected of a material with such an impressive level of ductility.
While the presenter intended his comments to be a criticism of PC, my interpretation of the disparity between frequency of use and frequency of failure are not so much a reflection of the polymer itself as they are an illustration of the difference between the impressive short-term properties of the material and some of the vulnerabilities that do not become apparent until time and the environment act on it.
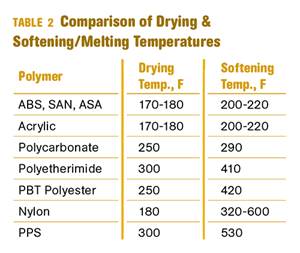
One of the initial weaknesses discovered through experience in the real world of small-appliance applications was the long-term susceptibility to hydrolytic degradation. In the short term, the high glass-transition temperature of polycarbonate (154 C/309 F) means that the material will maintain its physical properties when exposed to boiling water. PC’s sensitivity to the effects of excess moisture in the molten state, and the need for drying the resin prior to processing, were well understood early on.
However, the long-term effects of exposure to water when the material was in the solid form were not well characterized. The toughness, appearance, and dimensional stability of the material made it attractive to the small-appliance industry and one of the early applications was in coffee pots. These looked great on the store shelf and even for a while in the kitchen. But it was not long before these items began to crack due to the degradation that occurred from repeated exposure to hot water.

Despite its reputation for as-molded toughness, PC has a history of becoming brittle when exposed over time to high heat and humidity. Cracking in coffee pots is one example.
The electronics industry has discovered the same limitation in the material. While PC is perfectly serviceable in a wide range of applications in electronics, it does not perform well when subjected to a standard test conducted by the industry on various components. The test involves 1000 hr of exposure to a temperature of 85 C (185 F) and a relative humidity of 85%. Separately, this duration of exposure to either this temperature or this R.H. present no problems for PC.
But in combination they produce hydrolytic degradation that can be tracked by monitoring the melt-flow rate of the polymer during the tests. Studies have shown approximately a 70% increase in MFR from the as-molded part to parts conditioned at the high heat and humidity for 1000 hr, often with cracks developing in the parts. This response is greatly improved by using a PC-siloxane copolymer.
While PC is serviceable in a wide range of applications in electronics, it does not perform well when subjected to a standard test conducted by the industry on various components.
A second early discovery that caused some concern about the ductility of PC was a phenomenon known as critical thickness. This is a property that is not unique to PC. But because of its outstanding impact resistance, the behavior was more notable than it might have been in other materials.
Critical thickness refers to the significant decline in the toughness of a material when it is molded into thick-walled specimens. It arises from the internal stresses that develop within the part as the various layers undergo cooling from molten to solid at markedly different rates. This critical thickness has been measured at 0.250 in. (6.25 mm) in most general-purpose grades of PC but can be lower in low-molecular-weight compounds. In applications where toughness is paramount and a wall thickness greater than 0.250 in. is required, the desired functionality is typically obtained by laminating thinner sheets together to make a thicker structure.
However, it has been shown that the brittle behavior that causes the critical thickness phenomenon can be improved by cooling the polymer slowly when practical. In injection molding, high mold temperatures will substantially improve the toughness of thick structures. In addition, studies have shown that critical thickness is a property that is largely related to notched specimens. Well designed parts that are cooled slowly can be substantially thicker without losing their ductility.
The brittle behavior that causes the critical thickness phenomenon can be improved by cooling PC slowly when practical.
A third obstacle to wider use of PC became evident as the material encountered the real world of chemicals. Often, the subject of chemical resistance is the focus of these concerns. But a far greater problem is susceptibility to stress cracking, a phenomenon where cracking occurs due to the combined influence of stress and an applied chemical agent. The list of compounds that can produce this response in PC is extensive and includes ketones, esters, aldehydes, ethers, halogenated hydrocarbons and aromatic hydrocarbons. Many household cleaning compounds can stress-crack PC, and oils in many food products also cause a loss in its inherent ductility. Elevated temperatures aggravate the problem. Repeated dishwasher cycles are a well-documented cause of failure.
The BPA Issue
But perhaps the biggest challenge to the commercial image of PC is also the most controversial one, the concern over consumer exposure to bisphenol A, a monomer used to manufacture PC. Early indications that bisphenol A (BPA) might be an endocrine disruptor came from studies being done by endocrinologists who obtained anomalous data when using PC test tubes to hold their samples. While this discovery was made in the 1990s, the similarity of BPA to estrogen had been known since the 1930s. But it took a while for the concern over BPA to gain momentum.
Since then, an immense amount of information has been generated on the potential health effects of exposure to BPA. The Wikipedia page on BPA cites over 260 sources, perhaps the most completely cited subject on the website. The presence of some residual BPA in the PC polymer is inevitable. Under conditions where the polymer degrades, such as the aforementioned hydrolysis, additional BPA is produced.
The original concern involved bottles that contained baby formula. During sterilization of the formula, high heat and moisture are present, which potentially could produce BPA that could leach into the formula. While in adults, BPA is eliminated through a detoxification process in the liver, in infants and young children this pathway is not yet fully developed and BPA can accumulate. Because of the structure of the chemical, it can mimic the effects of estrogen, disrupting hormone balances and potentially resulting in infertility and early onset of puberty. As is often the case with health and environmental science, the concern over exposure to one segment of the population rapidly expanded into a hysterical reaction to BPA exposure for everyone.
The subject of risk of exposure to BPA has been studied globally, with many governments and scientific organizations weighing in. Many lengthy studies have concluded that exposure to BPA at current environmental levels remains acceptable. But at the same time, there have been major initiatives, some by the same chemical companies that proclaim its safety, to limit or eliminate its presence in various products. Other polymers not made from BPA, such as amorphous PET and other copolyesters, have benefitted from the hysteria, with suppliers of those materials proclaiming that products made from them are “BPA-free.”
The issue of BPA’s safety at this point is hardly settled science. The chemical industry has largely maintained that the compound is safe based on the fact that it has been around since the 1950s. On the other side of the discussion, almost every imaginable ailment that you can think of has been attributed to its presence in our environment.
Many lengthy studies have concluded that exposure to BPA at current environmental levels remains acceptable.
But PC is not even the most prominent source of BPA. Long before PC was invented, BPA was present in epoxy resins. These resins are frequently used as coatings in cans designed to hold food and beverages. But even here the BPA is bound in the polymer and takes time to migrate into the container contents. A more readily absorbable source is thermal and carbonless paper, used to produce receipts and tickets. Studies have shown that in this form it is so readily transferrable that paper money placed in contact with these items readily absorbs the BPA, becoming a major secondary source. So, regardless of the merits of the BPA-free argument, eliminating PC from our world would not stop the exposure.
Through all of these challenges, PC use continues to grow. We have learned a lot about the advantages and disadvantages of the material during its 60+ year history, and it would be difficult to imagine a world in which it did not exist. The copolyesters that have offered a BPA-free alternative lack the heat resistance of polycarbonate. The high-heat sulfones are very difficult to produce in a water-clear form, rapidly turn a darker color when exposed to UV radiation, and are generally more notch sensitive.
A polymer scientist at SABIC has developed a miscible alloy of amorphous PET and polyetherimide that has the same glass-transition temperature as polycarbonate, but it has not been commercialized. Perhaps someday there will be a return to a world where polycarbonate does not exist. But it is likely a long way off in the future and would require major breakthroughs in material research.
ABOUT THE AUTHOR: Michael Sepe is an independent materials and processing consultant based in Sedona, Ariz., with clients throughout North America, Europe, and Asia. He has more than 45 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 •mike@thematerialanalyst.com
Related Content
Why (and What) You Need to Dry
Other than polyolefins, almost every other polymer exhibits some level of polarity and therefore can absorb a certain amount of moisture from the atmosphere. Here’s a look at some of these materials, and what needs to be done to dry them.
Read MoreThe Effects of Temperature
The polymers we work with follow the same principles as the body: the hotter the environment becomes, the less performance we can expect.
Read MoreHow to Get Rid of Bubbles in Injection Molding
First find out if they are the result of trapped gas or a vacuum void. Then follow these steps to get rid of them.
Read MoreUnderstanding Strain-Rate Sensitivity In Polymers
Material behavior is fundamentally determined by the equivalence of time and temperature. But that principle tends to be lost on processors and designers. Here’s some guidance.
Read MoreRead Next
Tracing the History of Polymeric Materials: Polycarbonate
How polycarbonate came about, virtually simultaneously, through the efforts of two industry giants.
Read MoreTracing the History of Polymeric Materials: PVC & PVDC
How a class of materials based on chlorine chemistry became part of the landscape.
Read MoreTracing the History of Polymeric Materials: Teflon
The accidental discovery of Teflon.
Read More.jpg;width=70;height=70;mode=crop)