Tracing the History of Polymeric Materials: PET
How PET evolved from a material for fibers and fabrics to a force in packaging.
Last month’s column recounted a vital step in the commercialization of PET polyester. The accompanying figure shows one of several chemical processes used to create PET. This breakthrough involved using an organic acid containing an aromatic ring, terephthalic acid, to replace the aliphatic acids that were part of the original chemistry investigated by the Carothers’ team at DuPont. The aromatic ring boosted the performance of the resulting polymer, yielding a material with a melting point just 10° C (18° F) below that of nylon 66.
Incorporating the aromatic ring into the backbone structure of the polymer created a mixture of advantages and disadvantages. While the melting point of PET is slightly lower than that of nylon 66, the glass-transition temperature is 20° C (36° F) higher. In addition, the ester group, while polar, is much less hygroscopic than the amide group in nylon due to the absence of the hydrogen bond. And the aromatic structure reduces the susceptibility of the polymer to hydrolytic degradation, an important consideration in a material targeting fibers for clothing. Polyester exhibited less dimensional change with water absorption, which translated to a fabric that did not wrinkle when washed.
While DuPont may have passed on the initial development of polyester in favor of nylon, it obviously recognized the advantages of the new aromatic chemistry and in 1946 DuPont bought the rights to PET and developed its own product by 1950, trade named Dacron. In 1952 DuPont developed a thin-film form of the material that was trade-named Mylar. Eastman Chemical entered the market in 1958 with its own commercial offering, trade named Kodel.
Throughout the remainder of the 1950s and well into the 1960s, polyester became a dominant synthetic fiber before it fell out of favor due to complaints about comfort. Few polymers have as much of an association with changing societal tastes as polyester. The convenience of polyester fibers relative to natural fibers such as cotton and wool created a huge market in the 1950s and 1960s. This was followed by a period where polyester became associated with societal trends that made it appear out of step and obsolete (the disco scene comes to mind).
But while the material was losing market share in the world of fibers and fabrics, a new opportunity was on the horizon that capitalized on the amorphous form of the material. This was as an alternative to glass for water and carbonated beverage bottles. Here was a situation where crystallinity was not needed nor was it desired. The selling points were transparency and toughness, both of which required an amorphous polymer. Nathaniel Wyeth, a DuPont engineer, patented the technology for blow molding bottles from PET in 1973. The material proved to not only have the desired appearance and toughness needed for the application, it also possessed the strength and barrier properties needed for carbonated beverages.
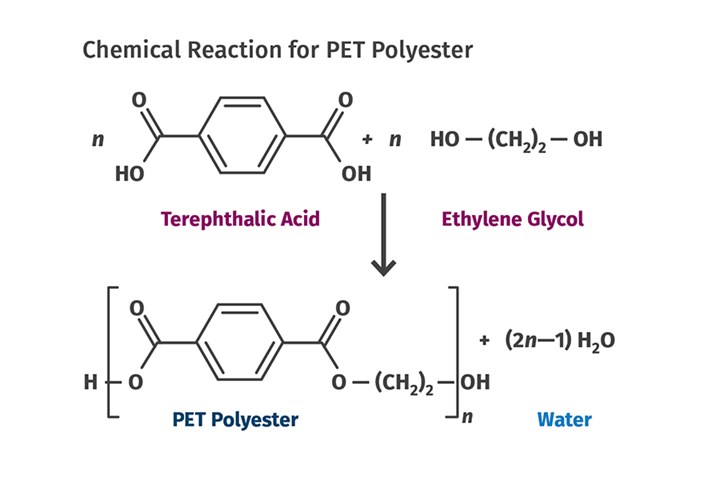
This is one of several chemical processes used to create PET polyester. This breakthrough involved using an organic acid containing an aromatic ring, terephthalic acid, to replace the aliphatic acids that were part of the original chemistry investigated by the Carothers team.
Ironically, DuPont did not benefit from Wyeth’s invention when it came to sales of raw material. DuPont lacked the ability to produce a polymer with a sufficiently high molecular weight for the bottle market. Both impact performance and barrier properties required this high molecular weight, and competitors such as Goodyear and Eastman brought on the capacity needed to satisfy demand.
A story regarding Wyeth’s early experiments in blow molding the polymer illustrates the difference between the properties of crystallized PET and amorphous PET. When Wyeth first attempted to blow mold a bottle from the material, the bottle shattered into small shards because the polymer had crystallized during the reheat process, making it very brittle. The importance of heating the material only slightly above the glass transition before blowing a bottle is well understood today, but the behavior of PET was still being learned in the early 1970s, and while crystallization was necessary for use of the material in fabric, it was not at all desired in bottles. Today, the systems that preheat molded preforms carefully control the temperature in order to avoid crystallization. A walk through a bottle blow molding plant will usually reveal a gaylord of scrap preforms that exhibit a cloudy appearance, a sign that the undesirable crystallization has started.
The behavior of PET was still being learned in the early 1970s ,and while crystallization was necessary for use of the material in fabric, it was not at all desired in bottles.
One of the consequences of the presence of the aromatic ring in the PET polymer backbone was a slower rate of crystallization than was typical in nylons. Because PET was used primarily in fibers, processing the material allowed for the achievement of an appropriate level of crystallization with all the associated beneficial properties. But the crystallization process was slow for the growing world of injection molded products. So, while nylon became an increasingly popular material for injection molded articles in the 1950s to the 1970s, PET remained a material for fibers and film, where the process of crystallization was slower but could be controlled through the manufacturing process.
In the amorphous state, PET polyester was relegated to applications that could accommodate the limited mechanical performance of a material with a glass-transition temperature of 80-85 C (176-185 F). Parts are injection molded in amorphous PET, but the application temperature is limited to about 65-70 C (149-158 F). The properties of amorphous PET present another challenge that can limit the maximum use temperature to an even greater degree. Because amorphous PET has one of the lowest glass-transition temperatures of any commercial amorphous polymer, it is susceptible to a phenomenon known as physical aging.
This was discovered in the early days of the plastic bottle industry when bottles stored at elevated temperatures exhibited a substantial decline in ductility in a matter of weeks. At the time there were only two mechanisms that were known to contribute to a decline in toughness—crystallization and polymer degradation. Neither of these could explain the loss in impact resistance of the PET bottles. But in 1978, L.C.E. Struik published a landmark paper outlining the mechanism of physical aging, a process in amorphous polymers that involves the collapse of the polymer chains into the free volume that exists between the chains. This creates stronger intermolecular forces that result in an increase in strength and stiffness but a decline in ductility over time.
Today amorphous PET accounts for the majority of PET resin consumed, due to over half a trillion bottles produced annually.
The rate at which physical aging occurs is dependent upon temperature, and studies by Eastman have shown that with each increase of 10° C the rate of physical aging in amorphous PET increases by a factor of nearly 10 (9.8 to be precise). My own experiments using accelerated aging techniques have confirmed this. And since the relationship is exponential, an increase of 30° C will increase the rate of physical aging by 9.83 or 941. So, a change in mechanical properties that might take a few years at room temperature can occur in a matter of days in a hot warehouse.
Today amorphous PET accounts for the majority of PET resin consumed, due to over half a trillion bottles produced annually. The ability of the polymer to be either amorphous or semi-crystalline is illustrated by the fact that much of the recycled PET from bottles is converted to fiber for clothing and other fabrics. But for applications that require heat resistance, such as under-the-hood environments, PET must be semi-crystalline. And most of these types of parts are produced by injection molding. This created problems for those wishing to use PET since the slow crystallization rate of the polymer resulted in long cycle times and the need for very high mold temperatures.
In 1978, DuPont solved the problem of slow crystallization through nucleation using a combination of additives and fillers to introduce a line of products known commercially as Rynite. But almost a decade earlier, another type of polyester had been introduced that did not exhibit the problems with slow crystallization and had already made inroads into the engineering thermoplastics market. This was PBT, a material with a structure similar to PET. The ability to achieve faster crystallization was due to a small change in the molecular structure that brought with it some compromises. PBT also revealed the potential for a remarkable level of versatility in polyesters, a topic we will cover in our next installment.
ABOUT THE AUTHOR: Michael Sepe is an independent materials and processing consultant based in Sedona, Ariz., with clients throughout North America, Europe, and Asia. He has more than 45 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 •mike@thematerialanalyst.com
Related Content
Let's Take a Journey into the World of Molding Thermosets – Part 1
There are many fundamental differences between thermosets and thermoplastics, from the way raw materials are furnished to the molder and the process in which parts are molded.
Read MorePrices Up for PE, ABS, PC, Nylons 6 and 66; Down for PP, PET and Flat for PS and PVC
Second quarter started with price hikes in PE and the four volume engineering resins, but relatively stable pricing was largely expected by the quarter’s end.
Read MoreAugust 2025: Prices for the 5 Commodity Resins Flat to Slightly Up
Barring weather and/or geopolitical production and supply disruptions, prices are generally steady.
Read MorePrices of All Five Commodity Plastics On the Way Up
Despite earlier anticipated rollover in prices for most of the volume commodity resins, prices were generally on the way up for all going into the third month of first quarter.
Read MoreRead Next
Tracing the History of Polymeric Materials: More on Polycarbonate
The industry has learned a lot about the advantages and disadvantages of polycarbonate during its more than 60-year history, and it would be difficult to imagine a world in which it did not exist.
Read MoreTracing the History of Polymeric Materials: How Nylons & Polyesters Connected
The history of nylons and polyesters are intertwined, and it takes some knowledge of chemistry to understand why.
Read MoreTracing the History of Polymeric Materials: Polycarbonate
How polycarbonate came about, virtually simultaneously, through the efforts of two industry giants.
Read More
.jpg;width=70;height=70;mode=crop)