Tracing the History of Polymeric Materials: Aliphatic Polyketone
Aliphatic polyketone is a material that gets little attention but is similar in chemistry to nylons, polyesters and acetals.

Shell’s Carilon was the first commercial aliphatic polyketone. It appeared in 1996 with bearing properties, wear resistance and chemical and hydrolysis resistance that challenged nylons, polyesters and acetals. It was discontinued in 2000, but Hyosung of Korea introduced a similar material in 2015. (Photo: Shell Chemical Co.)
Nylons, polyesters and acetals form a family of semi-crystalline polymers that represent the mid-range rungs on the price/performance ladder. They fit between the semi-crystalline commodity materials like polyethylene and polypropylene and the high-performance polymers such as polyphenylene sulfide (PPS). As we have seen, nylons, polyesters and acetals have interesting similarities in chemistry and performance while maintaining distinct places in the industry. Another material that fits into this family and gets little attention is known as aliphatic polyketone, sometimes abbreviated as PK or POK.
This is not to be confused with the very high-performance polymers polyetheretherketone (PEEK) and polyetherketone (PEK), which employ a highly aromatic backbone structure. The term aliphatic refers to a chain structure that does not employ aromatic rings, the six-sided structures that are an essential part of polymer chemistry. Figure 1 shows the chemical structure of the aliphatic and aromatic varieties of polyketone. The absence of the six-member rings in the aliphatic backbone results in a lower level of mechanical and thermal performance. However, it increases the chain mobility necessary for achieving a crystalline structure. PEEK was developed in 1978 while the aliphatic variety, was not commercialized until 1996. As usual, the history of development starts much earlier.
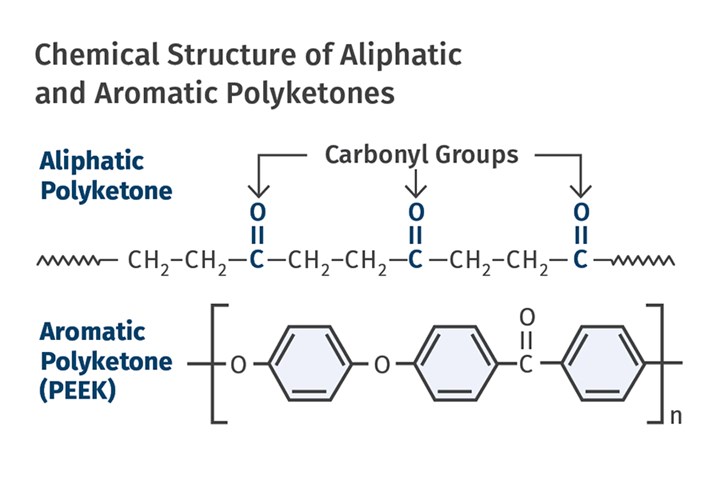
FIG 1 Absence of the six-member aromatic rings in the aliphatic backbone results in a lower level of mechanical and thermal performance. However, it increases the chain mobility necessary for achieving a crystalline structure.
Laboratory experiments that developed aliphatic polyketone polymers were conducted as far back as the 1940s and early 1950s, using nickel-based catalysts that required the use of very high pressures and temperatures. More developed catalysts were patented in the 1970s, allowing for easier polymerization of aliphatic polyketones.
Early aliphatic polyketones could not be melt processed because of poor thermal stability.
But as with the acetals we discussed previously, these materials could not be melt processed because of poor thermal stability. In this case the problem was an excessive level of residual catalyst in the resins. In the 1980s, improved palladium-based catalysts were investigated that led to the early stages of the research that would result in commercial development. This finally culminated in the introduction in 1996 of a line of commercial products by Shell, the holder of the patents, under the tradename Carilon.
The simplest version of this material consists of an alternating sequence of ethylene and carbonyl (C=O) groups, as shown in the top image of Fig. 1. It is essentially a copolymer of ethylene and carbon monoxide. The magic of the catalyst improvements provided for greater control over the arrangement of these two groups in the chain. Ethylene has a non-polar chemistry; however, the carbonyl group is decidedly polar, resulting in a material with higher strength, stiffness, and heat resistance that is a function the percentage of carbonyl functionality. The melting point of aliphatic polyketones tops out at about 260 C (500 F) for a 50/50 mix of the ethylene and carbonyl groups.
However, the commercial offerings are engineered to have melting points in the range of 200-220 C (392-428 F). The associated glass-transition temperatures are 5-15 C (41-59 F), just below room temperature. These properties can be adjusted by adding a third monomer, propylene, resulting in a structure like the one shown in Fig. 2. The addition of propylene monomer provides another tool for adjusting the crystallinity and melting point of the polymer, with increasing levels of propylene reducing both of these properties.
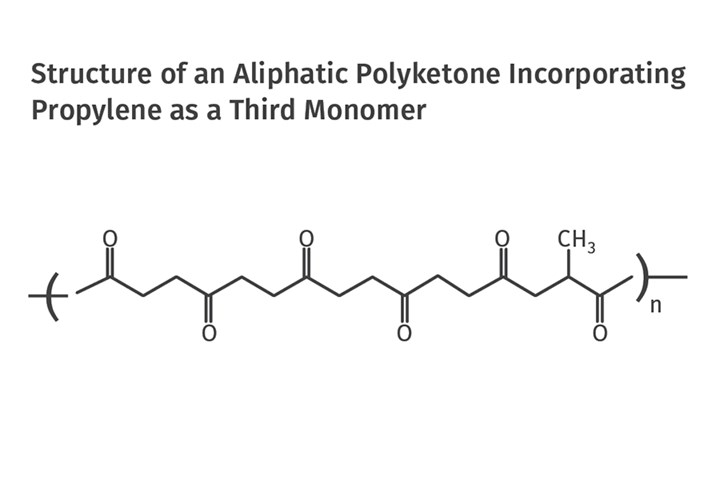
FIG 2 Commercial offerings of aliphatic polyketone have melting points in the range of 200-220 C and glass-transition temperatures of 5-15 C, just below room temperature. But these properties can be adjusted by adding a third monomer, propylene, resulting in a structure like the one shown here.
The result of incorporating the carbonyl functionality into what is otherwise a polyethylene chain produces a very interesting set of properties that give aliphatic polyketones a unique place in the market. The combination of polarity and crystallinity produces a very hard surface that provides very good bearing properties. Wear resistance is superior to that of acetals, despite the somewhat higher coefficient of friction of aliphatic polyketones. The distinctive feature of aliphatic polyketone chemistry is the presence of polar groups without introducing the oxygen into the polymer backbone.
The other three polymer families—nylons, polyesters, and acetals—all incorporate oxygen or nitrogen in the backbone. This makes them susceptible not only to moisture absorption but also to hydrolysis, a process that breaks polymer chains, causing degradation and property loss. While the polar carbonyl groups in the aliphatic polyketones do result in some moisture absorption, the carbon-only backbone produces a material with superior resistance to hydrolysis. It also provides improved resistance to a wide range of chemicals such as chlorides, aldehydes, weak acids and bases, and halogenated hydrocarbons.
Oxygen permeation in aliphatic polyketones is comparable to PVDC.
In addition, the chemical structure provides for excellent barrier properties for oxygen and hydrocarbons. Oxygen permeation in aliphatic polyketones is comparable to PVDC. This provides for excellent performance in applications like fuel-handling systems and some packaging. The barrier properties of aliphatic polyketones compare favorably with ethylene-vinyl alcohol copolymers (EVOH).
Mechanically, POK materials tend to have better impact resistance at room temperature than acetals, dry-as-molded nylons, and PBT polyesters. The tradeoff is that modulus of the material is lower than these other polymers. The modulus of unfilled POK is 1600 MPa (232 kpsi) while acetals range from 2800 to 3100 MPa (400-450 kpsi). Therefore, a direct replacement to take advantage of superior wear or barrier properties can be challenging if load-bearing performance is important and it may be necessary to add fillers or reinforcements. This, in turn, alters the shrinkage of the material, making it necessary to construct new molds.
The commercial history of aliphatic polyketones is unusual. While Shell introduced the material in 1996, by 2000 it stopped production, citing disappointing demand. Because it was the sole supplier of the materials, those who had adopted them were left without any alternatives. I recall having several conversations with end users, many of them producers of fuel systems, regarding potential alternatives. It is a testament to the uniqueness of this class of materials that such alternates simply did not exist. Tubing manufacturers had to return to multi-layer systems that had been used before the introduction of Carilon to achieve the same barrier characteristics. Acetals had to be modified with silicones and other additives to provide options that approached the wear resistance of the POK materials.
While the rationale for withdrawal from the market was poor demand, there was a lot of conversation within the industry regarding problems with process stability. The presence of a large number of carbonyl groups within the polymer chain provides the potential for crosslinking, and this chemical reactivity can be triggered by a wide variety of constituents. These include sizing agents on glass fibers, ingredients in colorants, and interaction with other polymers, including nylons and acetals. Studies have shown that introduction of the POK materials into a machine that was not properly purged can result in crosslinking at a rapid rate. This is an obvious problem for processors.
Introduction of POK into a machine that was not properly purged can result in crosslinking at a rapid rate.
Other studies have shown that one carbon black can be compatible with the POK materials while another seemingly equivalent carbon black results in crosslinking. Understanding the reactivity of the polymer to the wide range of ingredients that can be introduced to commercial compounds required a significant level of investigation and research, and conducting this learning curve on the manufacturing floor blunted enthusiasm for the material’s properties.
Nevertheless, POK was not dead. In 2003 a Korean company, Hyosung, began research and development on a POK material using the Shell patents, which Shell had donated to a research firm when it withdrew from the business. Not until 2015 did Hyosung introduce a commercial line of POK materials for extrusion and injection molding. Whether the reactivity problems that can lead to crosslinking have been solved or are now better understood so that they can be avoided is not clear. But the unique properties of the material make it a worthwhile addition to the market, so we can hope that this time around, the material will be a success in the manufacturing arena as well as in the laboratory.
In our next installment, we will take a historical step back and return to the amorphous polymer realm with a look at the development of acrylic.
ABOUT THE AUTHOR: Michael Sepe is an independent materials and processing consultant based in Sedona, Ariz., with clients throughout North America, Europe, and Asia. He has more than 45 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 • mike@thematerialanalyst.com
Related Content
Polyethylene Fundamentals – Part 4: Failed HDPE Case Study
Injection molders of small fuel tanks learned the hard way that a very small difference in density — 0.6% — could make a large difference in PE stress-crack resistance.
Read MorePolymer Showdown — PC/ABS vs. PC/PBT — May the Best Material Win
First in a series, experts from plastics engineering consultancy The Madison Group will pit leading thermoplastics against each other to see how they differ in processing characteristics, chemical resistance, thermal and mechanical performance, and more.
Read MoreThe Fundamentals of Polyethylene – Part 2: Density and Molecular Weight
PE properties can be adjusted either by changing the molecular weight or by altering the density. While this increases the possible combinations of properties, it also requires that the specification for the material be precise.
Read MoreFundamentals of Polyethylene – Part 5: Metallocenes
How the development of new catalysts—notably metallocenes—paved the way for the development of material grades never before possible.
Read MoreRead Next
Tracing the History of Polymeric Materials: The Differences Between Nylons & Polyesters
In many respects, nylons and polyesters appear to be interchangeable. But there are interesting differences in the properties of these two families that arise from their chemical structures.
Read MoreTracing the History of Polymeric Materials: Acetal
The road from discovery in the lab to commercial viability can be long, and this was certainly the case for acetal polymers.
Read MoreTracing the History of Polymeric Materials: Polyesters
Beyond PET, PBT and their analogues, development of polyester chemistry led to unsaturated thermosetting resins, copolyester thermoplastic elastomers, liquid-crystal polymers and, most recently, biopolymers.
Read More
.jpg;width=70;height=70;mode=crop)