Tracing the History of Polymeric Materials: The Commercialization of Acrylic
We covered the invention of acrylic in our last installment. Here, we discuss its commercial development.

Acrylic-sheet greenhouse glazing takes advantage of the material’s transparency and resistance to weathering. (Photo: Arkema)
Despite the fact that the processes for making esters of acrylic acid were worked out at Rohm & Haas by 1921, economic conditions and the desire of the company to keep the developments secret stalled the path to commercialization of acrylic polymer. At the time, Rohm & Haas was a relatively small company by chemical industry standards, and its income relied heavily on the leather tanning product that Rohm had created in the early days of the company, known as Oropon.
Due to the combination of hyperinflation in Germany’s currency in the mid-1920s, the subsequent worldwide depression, and the end of patent protection of Oropon, the price of the product fell nearly 50% between 1925 and 1933. Consequently, while experimental research was directed toward polymers, the need for income dictated that some of the efforts were diverted to by-products from the acrylic ester synthesis. A key product that kept the company in the black was fire extinguishers filled with ethylene bromide.
The ability of Rohm & Haas to move forward with the production of acrylic polymers was facilitated in 1927 when the company entered into an agreement with I.G. Farbenindustrie to obtain ethylene cyanohydrin. This reduced the number of steps in Walter Bauer’s ethylene process and eliminated the costs that Rohm & Haas was incurring in making the raw materials in house. The one-pot process then used the ethylene cyanohydrin to make their monomer, ethyl acrylate.
Initially, the process for converting ethyl acrylate into a polymer was not well controlled. Unlike the rigid materials we normally think of when we consider modern acrylics, the early polymers were relatively flexible. This made them useful as electrical insulation, coatings for textiles, lacquers for use in dyes, and bonding agents for use in adhesives—all applications that acrylic is still used in today. These materials were given the trade name Plexigum in 1927. The origin of this name seems to be lost to history, although it may have been a derogatory term conferred by Otto Rohm over his frustration regarding the properties of these materials.
The first acrylic-bases safety glass was adopted for safety goggles, gas masks and auto windscreens.
In 1928, Bauer and another chemist, Adolf Gerlach, discovered that they could polymerize the acrylic ester between panes of glass. The resulting material was stiffer and created such a strong bond between the glass plates that they could not be separated. Attempts to do so made it obvious that the polymer layer prevented the shattering of the glass and unlike the celluloid that was available at the time, this new polymer did not turn yellow when exposed to sunlight. Rohm & Haas had their first plastic product, created as usual through a series of coincidences, trade named Luglas in 1929. Bauer and Gerlach shared the patent.
This first safety glass was adopted for safety goggles, gas masks and car windscreens. But the details of the polymerization process were still being worked out, and the initial approach of polymerization in solvents turned out to be a chain reaction that could be explosive. In the meantime, chemists at I.G. Farbenindustrie developed a method for emulsion polymerization that made the reaction much safer and more manageable. They received the patent for this development in 1930. I.G. Farbenindustrie had little interest in safety glass. Instead, they were focused on fibers for making artificial silk. But the advantages of the emulsion polymerization process were obvious and critical in allowing Rohm & Haas to produce the rigid polymer profitably.
In 1932, Rohm & Haas and I.G. Farbenindustrie entered into an agreement whereby Rohm & Haas was granted the right to share the emulsion polymerization patent while I.G. Farben took over monomer production, including the coveted one-pot process as well as some of the polymer production. Only Luglas was excluded from this arrangement.
In 1933, Rohm and Haas discovered that they could polymerize methyl methacrylate between sheets of glass to create a sheet material that was trade named Plexiglas. By 1936, this process was being used to form the material into a structure that could be used to substitute for glass and was marketed as a safety glass for car windows, largely supplanting Luglas in the market. In the intervening period between 1928 and 1936, chemists at Imperial Chemical Industries (ICI) discovered the polymerization of methyl methacrylate and created a product they designated “acrylic glass,” trade named Perspex.
During the same time period, DuPont came up with its version that was trade named Lucite. A year after the introduction of Plexiglas, molding powders were commercialized for melt processing by extrusion and injection molding. Despite these concurrent developments, there appears to have been no flurry of patent disputes and litigation among these companies. This may be attributable to the unstable political situation in Europe at the time.
The acrylic chemistry produced a material with outstanding transparency, high surface hardness, high strength and stiffness, dimensional stability, excellent electrical insulating properties, and among the best levels of resistance to degradation from ultraviolet light of any known polymer. In our modern era, where transparent materials like polycarbonate are available, it may seem laughable that acrylics were considered to be tough.
But when compared with glass, acrylics were an order of magnitude more impact resistant while reducing weight by almost 60%. This led to rapid adoption of the materials in applications like canopies, aircraft windshields, skylights, windscreens, and outdoor signage. The timing of the introduction of the material dovetailed fortuitously with the advent of the second World War, which led to applications in the military such as submarine periscopes and gun turrets.
When compared with glass, acrylics were an order of magnitude more impact resistant while reducing weight by almost 60%.
Consumer applications included aquariums, bathtubs and countertop materials that take advantage of the dimensional stability and hardness of the material as well as its ability to accept color and resist yellowing. The introduction of polycarbonate some 20 years later did bring a new level of impact resistance to the world of polymers and it raised the upper end-use temperature of transparent polymers by approximately 50°C. However, even with the addition of additive packages designed to extend uv resistance, polycarbonate still cannot match the stability of acrylics to the effects of outdoor weathering, as any automotive lighting engineer will tell you. Even with coatings applied to polycarbonate to slow down the effects of outdoor weathering, acrylics still outperform polycarbonate in this respect.
Because impact resistance has always been relatively poor for acrylics, a lot of attention has been given to improving this property. This can be achieved in several ways, including increased molecular weight, modification of the methyl methacrylate chemistry for a portion of the polymer structure, and addition of impact modifiers. This last approach can reduce uv resistance and also impairs the excellent light transmittance of the base polymer. Acrylic polymers can be used in blends with materials like ABS or in copolymers with styrene to provide certain property profiles. Thin acrylic sheets can be formed over substrates of less uv-stable materials like ABS to provide the desired combination of toughness and weatherability.
While acrylics are generically referred to as PMMA, commercial acrylics are typically copolymers that employ modifications to the methyl methacrylate chemistry in order to adjust mechanical and thermal properties. Pure PMMA was originally made by free-radical polymerization, which produces a polymer with an atactic structure. Pure atactic PMMA has a glass-transition temperature (Tg) of approximately 120°C (248°F), while most commercial acrylics exhibit Tg’s of 90-105°C. This results from the chemistry modifications made to commercial materials. Varying the chemistry by substituting larger alkyl groups allows for the adjustment of the thermal and mechanical properties of the material while still retaining the transparency and outstanding uv stability. Using methacrylic acid as a comonomer will raise the softening temperature of the material, and acrylic-imide copolymers also provide a substantial improvement in thermal performance, sometimes equaling the heat resistance of polycarbonate.
Later, a different type of polymerization process known as anionic polymerization was found to yield both isotactic and syndiotactic versions of the polymer. Atactic, isotactic and syndiotactic are terms describing the way pendent groups are arranged on a polymer backbone, referring to which side of the polymer chain the pendant groups are on. All of these forms of PMMA are amorphous and therefore still transparent, but their heat resistance varies greatly. The Tg of isotactic PMMA is only 45°C (113°F) while that of syndiotactic PMMA is 130°C (266°F). Combining these different structures offers another approach to tailoring properties.
In our next installment, we will look at the development of a unique material that has been alternately referred to as polyphenylene oxide and polyphenylene ether.
ABOUT THE AUTHOR: Michael Sepe is an independent materials and processing consultant based in Sedona, Ariz., with clients throughout North America, Europe, and Asia. He has more than 45 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 • mike@thematerialanalyst.com
Related Content
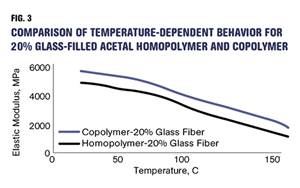
How Do You Like Your Acetal: Homopolymer or Copolymer?
Acetal materials have been a commercial option for more than 50 years.
Read MorePrices for All Volume Resins Head Down at End of 2023
Flat-to-downward trajectory for at least this month.
Read MoreTracing the History of Polymeric Materials: Aliphatic Polyketone
Aliphatic polyketone is a material that gets little attention but is similar in chemistry to nylons, polyesters and acetals.
Read MoreWhat is the Allowable Moisture Content in Nylons? It Depends (Part 1)
A lot of the nylon that is processed is filled or reinforced, but the data sheets generally don’t account for this, making drying recommendations confusing. Here’s what you need to know.
Read MoreRead Next
Tracing the History of Polymeric Materials: Aliphatic Polyketone
Aliphatic polyketone is a material that gets little attention but is similar in chemistry to nylons, polyesters and acetals.
Read MoreTracing the History of Polymeric Materials: The Differences Between Nylons & Polyesters
In many respects, nylons and polyesters appear to be interchangeable. But there are interesting differences in the properties of these two families that arise from their chemical structures.
Read MoreTracing the History of Polymeric Materials: Acetal
The road from discovery in the lab to commercial viability can be long, and this was certainly the case for acetal polymers.
Read More
.jpg;width=70;height=70;mode=crop)