Tracing the History of Polymeric Materials, Part 28: Making LCP's Melt Processable
Liquid-crystal polymers based on a single monomer produces a polymer with a very high melting point.This presents two problems. Here’s how they were solved.
As we mentioned in Part 27 of this series in March, liquid-crystal polymers (LCPs) based on the single monomer, p-hydroxybenzoic acid produce a polymer with a melting point of approximately 500°C (932°F). This presents two problems. First, most melt-processing equipment is limited to a temperature of 421°C (800°F). Second, even if our equipment could reach these extreme temperatures, the polymer would undergo thermal degradation before it reached the melting point, making it similar to Kevlar, an LCP based on polyamide chemistry that is not melt processable.
Making the material accessible to mainstream manufacturing involved adjusting the chemistry to produce manageable melting points. This was achieved by incorporating comonomers into the polymer backbone to reduce the melting point of the resulting polymer. An example of this is hydroxynaphthenoic acid, which produces the chemistry shown in Fig. 1. By adjusting the ratio of these two monomers, the transition temperature from solid to a system that flows like a liquid can be altered to meet different performance profiles.
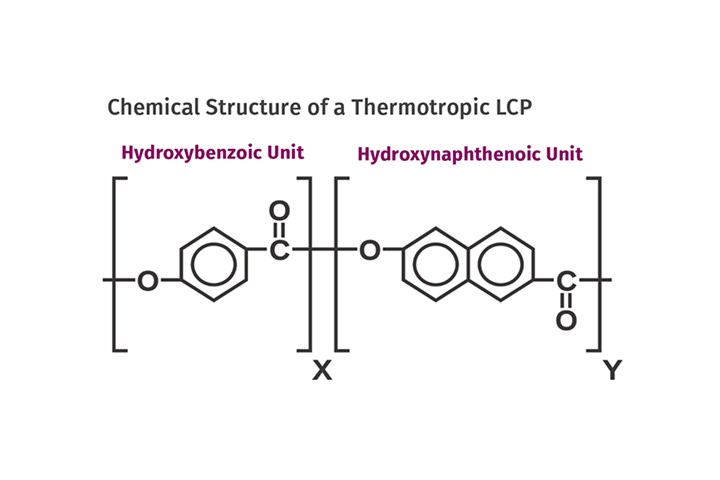
(FIG 1) An example of this is hydroxynaphthenoic acid, which produces the chemistry shown here. (All Images: Mike Sepe)
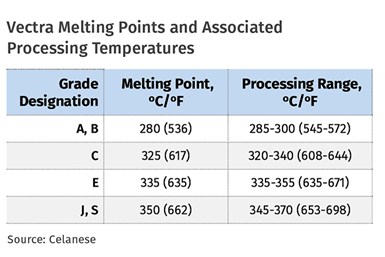
An example of this can be seen in a commercial product line such as the Vectra materials from Celanese. The accompanying table shows a variety of grades and their associated melting points and related melt-temperature processing ranges as published in the literature. Higher-heat grades employ a higher percentage of the hydroxybenzoic units in the polymer backbone.
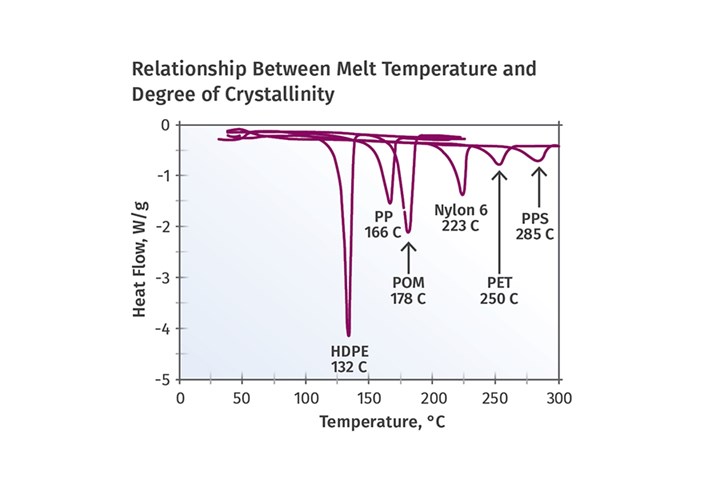
Of course, the term melting point must be used carefully in reference to the behavior of LCPs since, by definition, the transition from solid to molten does not involve a true breaking up of the crystal structure. This can be observed when studying the phase change associated with the melting of a semi-crystalline polymer. Figure 2 shows the melting behavior for a variety of semi-crystalline polymers. In each case the melting process is clearly identified by a curve that departs from the baseline and then returns to the baseline once the melting process is complete. The melting point is identified as the temperature at which the peak occurs, and the energy required to melt the crystals, known as the latent heat of fusion, is measured by the area under the curve.
FIG 2 Melting behavior for a variety of semi-crystalline polymers: In each case the melting process is clearly identified by a curve that departs from the baseline and then returns to the baseline once melting is complete. The melting point is identified as the temperature at which the peak occurs; the energy required to melt the crystals (latent heat of fusion) is measured by the area under the curve.
As an example, the HDPE in this figure has a melting point of 132°C (270°F) and the heat of fusion is approximately 220 J/g. This represents the energy required to convert the solid HDPE to a fluid and it includes a requirement in addition to the energy needed to increase the temperature of the material to the desired processing temperature. It should also be noted that when the material cools back to the solid state, this energy comes back out of the material and must be managed by the cooling system in the mold. This is one of the reasons that cycle times in semi-crystalline materials tend to be longer than expected.
Parts molded in LCPs should provide for much shorter cycle times than the same parts molded in a conventional semi-crystalline material.
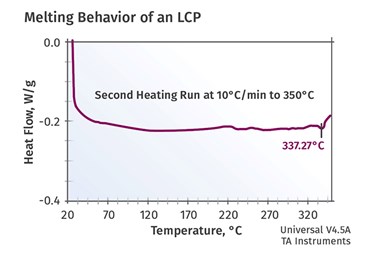
FIG 3 Measurement of the “melting” behavior of an LCP using the same instrumentation employed in Fig. 2.
Figure 3 shows the measurement of the “melting” behavior of an LCP using the same instrumentation employed in Fig. 2. The event is barely visible at 337°C (639°F) and the energy associated with the transition is only 1 J/g, infinitesimal compared to the energy needed to melt a conventional semi-crystalline polymer. This leads to a lot of confusion within the industry about whether LCPs are semi-crystalline or amorphous. They do, indeed, possess a crystal structure. But this structure does not truly melt when the material is converted from solid pellets to molten flowing polymer and almost no energy is required to effect the change of state. By the same token, upon cooling to the solid state almost no additional energy is released.
Consequently, parts molded in LCPs should provide for much shorter cycle times than the same parts molded in a conventional semi-crystalline material. In addition, because the change from the molten state to the solid state does not involve the typical significant phase change, a lot of the problems that we encounter when molding parts from semi-crystalline materials do not occur with LCP. These include high levels of shrinkage, anisotropic shrinkage, and localized differences in density within the structure that all can result in warpage and dimensional variability. This comparative stability is more typical of amorphous polymers.
One of the most notable characteristics of LCP materials is their low melt viscosity. Even with the addition of significant amounts of filler and reinforcing fiber, these materials possess perhaps the lowest viscosity of any molten polymer at high shear rates, allowing for the filling of very thin sections. In addition, this class of materials exhibits inherent flame retardance and very high relative thermal indices. However, the high degree of alignment of the polymer chains and the fact that the molten polymer does not lose its crystal structure even when flowing means that the weld-line strength of the materials is among the lowest of all polymers.
Traditional data sheets only use one metric for evaluating elevated-temperature performance, the deflection temperature under load (DTUL or HDT). Because of the high solid-to-liquid transition temperatures of these materials and the fact that almost all grades are highly filled, the values for this property are very impressive. Values for 30% glassfiber-reinforced grades are typically above 205°C (401°F) and sometimes exceed 260°C (500°F). However, an examination of the temperature-dependent behavior of the materials by dynamic mechanical analysis (DMA) shows that LCPs do not display extended temperature ranges where the modulus of the material is stable, as is typical with most other polymers.
One of the most notable characteristics of LCP materials is their low melt viscosity.
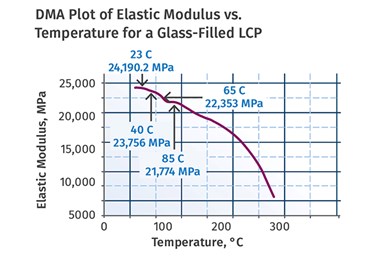
(FIG 4) LCP modulus declines steadily with temperature, and the rate of decline increases as temperatures rise.
Instead, as shown in Fig. 4, the modulus declines steadily with temperature and this rate of decline increases as temperatures rise. This translates into relatively poor creep resistance despite the high degree of stiffness exhibited by the materials at room temperature. This assessment was contested by suppliers of the materials in the early days. However, in later years blends of LCP with various nylons or PPS were commercialized to compensate for this weakness.
On the positive side, while LCP materials are primarily polyesters, the high degree of aromatic character in the polymer backbone prevents a well-known weakness in polyesters — a tendency to undergo hydrolysis. This is a useful characteristic for processors as well as for end users who require durability in environments where high heat and humidity are present.
The biggest barrier to the use of LCP is cost. But this is usually thought of solely in terms of cost per pound and can be a shortsighted approach. The low thermal barrier between solid and molten polymer results in the potential for very fast cycles when compared with potential competitors like glass-filled nylons, PET and PBT polyesters, and PPS. This will result in the ability to meet production requirements with smaller molds and lower cavitation.
This, in turn, translates to lower manufacturing costs and less variation due to potential imbalances in mold filling in a multi-cavity mold. When combined with the improved dimensional stability, predictability of shrinkage, resistance to warpage, and very low melt viscosity, these considerations can result in a lower total cost despite the higher price of the raw material.
ABOUT THE AUTHOR: Michael Sepe is an independent materials and processing consultant based in Sedona, Ariz., with clients throughout North America, Europe, and Asia. He has more than 45 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting and failure analysis. Contact: (928) 203-0408 • mike@thematerialanalyst.com
Related Content
Prices for All Volume Resins Head Down at End of 2023
Flat-to-downward trajectory for at least this month.
Read MoreWhat's the Allowable Moisture Content in Nylons? It Depends: Part 2
Operating within guidelines from material suppliers can produce levels of polymer degradation. Get around it with better control over either the temperature of the melt or the barrel residence time.
Read MoreScaling Up Sustainable Solutions for Fiber Reinforced Composite Materials
Oak Ridge National Laboratory's Sustainable Manufacturing Technologies Group helps industrial partners tackle the sustainability challenges presented by fiber-reinforced composite materials.
Read MoreTracing the History of Polymeric Materials: Polyphenylene Oxide
Behind the scenes of the discovery of PPO.
Read MoreRead Next
Tracing the History of Polymeric Materials, Part 25: Silicones
The long road to the development of silicone resulted in a chemistry that is remarkably versatile.
Read MoreTracing the History of Polymeric Materials, Part 26: High-Performance Thermoplastics
The majority of the polymers that today we rely on for outstanding performance — such as polysulfone, polyethersulfone, polyphenylsulfone and PPS — were introduced in the period between 1965 and 1985. Here’s how they entered your toolbox of engineering of materials.
Read MoreTracing the History of Polymeric Materials, Part 27: Liquid-Crystal Polymers
Liquid-crystal polymers debuted in the mid-1980s, but the history of the chemistry associated with this class of materials actually starts a century earlier.
Read More.jpg;width=70;height=70;mode=crop)