Tracing the History of Polymeric Materials: Polyphenylene Oxide
Behind the scenes of the discovery of PPO.
By the mid-1950s, polymer chemists had come to understand that there was a relationship between the stiffness of the molecular chains that make up the polymer backbone and the property known as the glass-transition temperature (Tg). In amorphous polymers, the Tg represents the softening temperature of the material, and maximum short-term use temperatures will typically be about 10-20oC below the Tg. Therefore, developing chemistries that maximize molecular stiffness represent an approach for extending the heat resistance of plastic materials.
Recognition of this relationship led to research in polymerizing phenols, chemicals that had a structure that could significantly increase upper temperature limits. Phenol was already the basis for phenolic, a thermosetting polymer that had been invented in 1909 by Leo Baekeland. But the focus of the work that began in 1956 was on thermoplastics, and the chemical process that resulted in the commercial success of these efforts is known as oxidative coupling, a novel reaction that does not fit neatly into the more common polymerization processes typically known as addition or condensation polymerization.
Figure 1 shows the chemical structure for the specific compound that became the monomer for the polymer known as polyphenylene oxide (PPO). Phenol is benzene, a six-carbon aromatic ring, with a hydroxyl (-OH) group substituting for one of the six hydrogen atoms on the benzene ring. In organic chemistry nomenclature, the carbon to which the hydroxyl group is attached is assigned to be the number 1 carbon, as shown in this figure. The other five carbons are numbered sequentially as we move around the ring clockwise. The two methyl groups attached to the number 2 carbon and the number 6 carbon complete the picture and give the molecule its name.
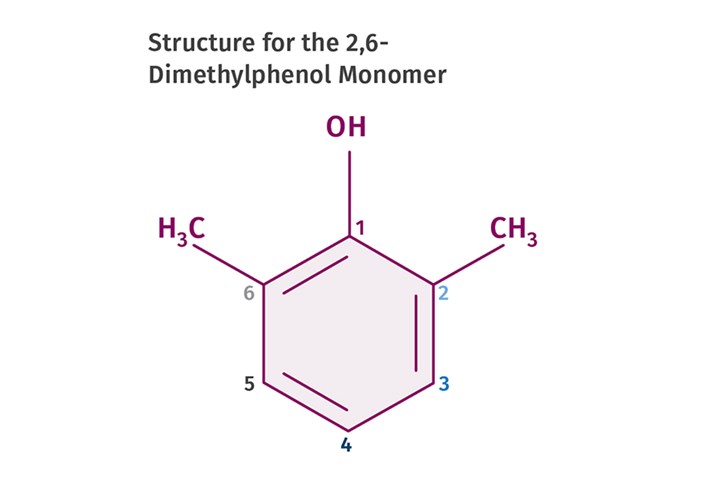
FIG 1 Phenol is benzene, a six-carbon aromatic ring, with a hydroxyl (-OH) group substituting for one of the six hydrogen atoms on the benzene ring. In organic chemistry nomenclature, the carbon to which the hydroxyl group is attached is assigned to be the number 1 carbon. The other five carbons are numbered sequentially as we move around the ring.
Traditionally, reacting a substituted phenol like this with itself resulted in what is called a dimer—two molecules joined together with a double bond at the number 4 carbons, with the oxygens remaining at the number 1 positions through a process known as carbon coupling. The resulting structure is shown in Fig. 2 and is called a diphenoquinone. This is the dimer (two monomer units linked together) and the reaction at this point is effectively over; no polymerization is possible.
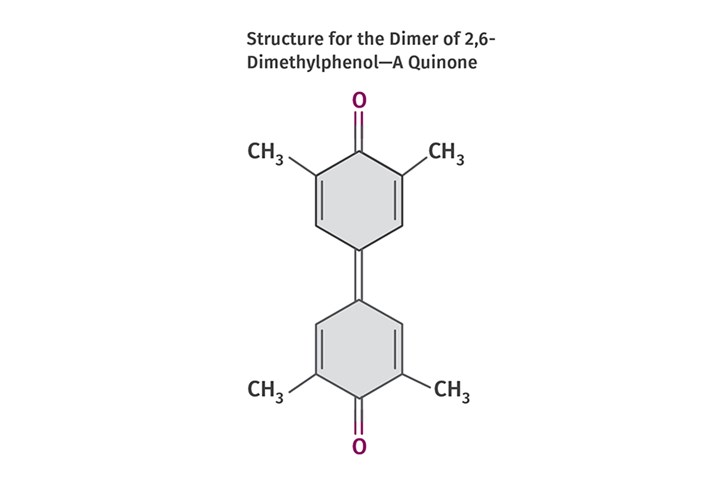
FIG 2 Reacting a substituted phenol with itself results in a dimer — two molecules joined with a double bond at the number 4 carbons with the oxygens remaining at the number 1 positions through a process known as carbon coupling. This is the resulting structure.
The alternative path is known as oxygen coupling, where the oxygen atom in the hydroxyl group of the monomer forms the link with the next monomer unit. This path leads to polymerization, resulting in the structure shown in Fig. 3. Chemists began exploring this possibility in 1956 and employed a variety of catalysts to promote the desired reaction. It was recognized that if a polymer could be created from a chemistry that was replete with aromatic rings, it would have outstanding mechanical and thermal properties. For three years various chemists experimented with different approaches that produced some degree of polymerization. But the resulting chain lengths were too short to provide good mechanical performance. As we have seen repeatedly, the key to turning a laboratory curiosity into a scalable commercial polymer was the correct catalyst.
If a polymer could be created from a chemistry that was replete with aromatic rings, it would have outstanding mechanical and thermal properties.
In 1959, Allan Hay, a chemist at GE Plastics, developed a reaction that involved “merely” passing oxygen through a solution of the 2,6-dimethyphenol in an organic solvent that contained an amine and a copper catalyst. The term merely is a direct quote from Hay’s paper, an elegant example of humility within the brilliance. This breakthrough produced a very high-molecular-weight product that had excellent mechanical properties. It was amorphous, but had a Tg of 218oC (424oF). Consider the historical context of this development. Polycarbonate, which was on the verge of commercialization, has a Tg of 153oC (307oF). Polysulfone, with a Tg of 187oC (368oF), was still half a decade away. And even the high-heat amorphous polymers like polyethersulfone and polyetherimide, which were developed in the 1970s and early 1980s, just match the heat resistance of the original polymer that Hay and his team created.
The selection of the right monomer turned out to be crucial. First, the placement of the methyl groups at the 2 and 6 positions allows for the development of a linear polymer. In addition, larger substituent groups hindered the carbon-oxygen coupling mechanism. For example, 2,6 di-tertbutyl phenol, a commonly used antioxidant, does not produce carbon-oxygen coupling. So, the simplicity and position of the methyl groups was important to the success of the polymerization process.
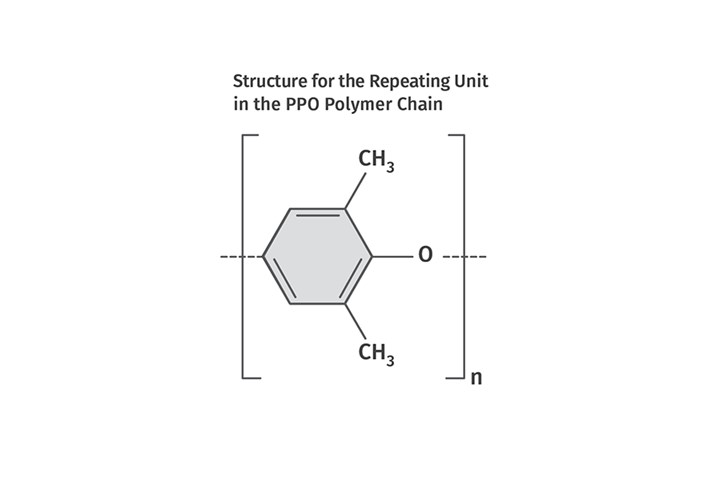
FIG 3 Oxygen coupling, where the oxygen atom in the hydroxyl group of the monomer forms the link with the next monomer unit, leads to polymerization, resulting in the structure shown here.
The polymer was initially called poly(phenylene oxide) or PPO to reflect the presence of the aromatic ring bonded to an oxygen atom in the repeating unit. In organic chemistry, groups attached to each other through a connection to an oxygen atom are classified as ethers. Therefore, later on, especially as other companies developed competing commercial offerings, the polymer became more commonly known as poly(phenylene ether) or PPE. But they are essentially the same material under two different naming traditions.
The viscosity of the melt was very high, making production of parts with even moderate flow paths nearly impossible.
The journey from initial discovery to commercialization was remarkably fast, with introduction to the market in 1960. Given the high heat resistance and excellent mechanical properties arising from the molecular structure, there was an expectation that the material would extend the capability of polymeric materials into previously unexplored territory, and a plant dedicated to its production was established in Selkirk, N.Y.
But there was a problem. As impressive as the properties of the material were, its processability left a great deal to be desired. The viscosity of the melt was very high, making production of parts with even moderate flow paths nearly impossible. And if the part could be made, the internal stresses within the material were so high that parts would spontaneously develop cracks just sitting in storage or on someone’s desk.
In 1988 I met Larry Nunnery. He had just purchased BMC Corp., a small company in St. Charles, Ill., that made unsaturated polyesters. In the years following, Larry turned this operation into a powerhouse and ignited a much-needed conversation within the plastics industry on the importance of thermoset materials. Before Larry became the owner of BMC, he had worked for GE Plastics. He related a story to me about calling on a customer who was having problems with cracking in parts molded from PPO. The customer was storing the parts in a dark room under the mistaken belief that the cracking was caused by the ultraviolet light coming from fluorescent bulbs in the office. But when he took Larry into this dark room and closed the door, Larry said you could hear the parts cracking as they sat on the shelves.
This was a serious threat to the development of the material. GE Plastics (purchased by SABIC in 2007) had invested significant funds in the Selkirk plant and they had a material that, at least on paper, was poised to conquer the world. But very few processors could convert the raw material to usable parts. The solution to this problem was, as we have seen so often, an accidental discovery by someone working closely with the material in the plant. We will take up that part of the story in our next installment.
ABOUT THE AUTHOR: Michael Sepe is an independent materials and processing consultant based in Sedona, Ariz., with clients throughout North America, Europe, and Asia. He has more than 45 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting and failure analysis. Contact: (928) 203-0408 • mike@thematerialanalyst.com
Related Content
How Much L/D Do You Really Need?
Just like selecting the extruder size and drive combination, the L/D should be carefully evaluated.
Read MoreAre Your Sprue or Parts Sticking? Here Are Some Solutions
When a sprue or part sticks, the result of trying to unstick it is often more scratches or undercuts, making the problem worse and the fix more costly. Here’s how to set up a proper procedure for this sticky wicket.
Read MoreUnderstanding Strain-Rate Sensitivity In Polymers
Material behavior is fundamentally determined by the equivalence of time and temperature. But that principle tends to be lost on processors and designers. Here’s some guidance.
Read MoreThe Strain Rate Effect
The rate of loading for a plastic material is a key component of how we perceive its performance.
Read MoreRead Next
Tracing the History of Polymeric Materials: Aliphatic Polyketone
Aliphatic polyketone is a material that gets little attention but is similar in chemistry to nylons, polyesters and acetals.
Read MoreTracing the History of Polymeric Materials: The Commercialization of Acrylic
We covered the invention of acrylic in our last installment. Here, we discuss its commercial development.
Read More.jpg;width=70;height=70;mode=crop)