Tracing the History of Polymeric Materials, Part 27: Liquid-Crystal Polymers
Liquid-crystal polymers debuted in the mid-1980s, but the history of the chemistry associated with this class of materials actually starts a century earlier.

This LCP from SABIC (LNP Konduit 8TF36E) is designed to address the stringent demands of burn-in test sockets (BiTS) used to stress-test double-data-rate (DDR) memory circuits. (Photo: SABIC)
More than 40 years ago, I was asked by a client to locate a material with the greatest available heat resistance. This was before the era of the online databases such as Prospector and Matweb, so this involved searching printed publications that consolidated the data sheets offered by the various material suppliers. My version of this resource was a large book with a soft, green, blue or red cover, depending upon the year of issue, published yearly by Plastics Technology as its Handbook & Buyers’ Guide.
At that time, the only metric for assessing this property was the deflection temperature under load, a property whose limitations I have written about extensively. Combing through the pages, I came across an unfamiliar class of materials identified as liquid-crystal polymers (LCP). There were very few entries in this group and the one with the most impressive claim to high heat resistance was a single grade from a company that was unfamiliar to me, Dart Industries. The grade, if memory serves, was called DH-1000, and it boasted a deflection temperature under load (DTUL) of over 700°F (372°C) and a melting point of 790°F (421°C).
Thumbing to the back of the reference, I found a contact phone number for Dart Industries and called it. The gentleman who answered the phone seemed reluctant to give many details about the material despite my obvious interest in using it for a specialty application. At one point he even asked me how I obtained his number. The conversation was not fruitful. But a few years later, a product line within the Tupperware company appeared on the market under the brand Ultra 21. It was quite a departure from the flexible polyolefin food storage products that Tupperware had long been known for.
These were pans for baking bread and cooking lasagna, large parts with a distinctive cream-color appearance, a great deal of rigidity, and the ability to withstand hours of exposure to oven temperatures up to 500°F (260°C) without deforming and while being appropriate for direct food contact at these temperatures. The wonder material turned out to be an LCP and at that time Tupperware was part of the Dart Industries family of companies. We discussed these materials briefly in the March issue of last year when covering the larger topic of polyesters.
This may have been the first commercial application of what are known as thermotropic, or melt-processable, LCPs and it was quite different from the applications that we associate with these polymers today. This initial melt-processable LCP required such a high melt temperature that it was at the limit of what was achievable on a standard injection molding machine. Ironically, these products avoided one of the biggest weaknesses of these materials, poor weld-line strength. All of these parts were center-gated, had a uniform and relatively thick nominal wall, and were solid from gate to end of flow.
Apparently, the Dart Industries material remained within the realm of the captive market, being made and provided specifically to satisfy cookware. But soon after, companies that are more familiar names in the world of polymers, like Celanese (Vectra), Amoco (Xydar), and DuPont (Zenite), emerged with product lines also classified as LCPs. The grades within these product lines were distinguished primarily by their melting points, which is controlled by the chemistry of the materials, and their strength and stiffness, which is determined by the type and amount of filler or reinforcement incorporated into the material. While this introduction occurred in the mid-1980s, the history of the chemistry associated with LCP starts a century earlier.
The unique shape of molecules capable of forming liquid crystals can come from the main chain structure or from side-chain structure.
The first person to observe the state of matter that today we call liquid crystals was Austrian botanist Friedrich Reinitzer. He was conducting experiments in 1888 on a substance known as cholesteryl benzoate, a chemical found in plants (see below). He observed when heating the substance that it exhibited two melting points. The solid crystals turned into a hazy liquid at 145°C (293°F) and then with additional heating the hazy liquid became transparent at 178°C (352°F). In the same year, the German physicist Otto Lehmann concluded that the cloudy fluid was a new state of matter that existed between the well-ordered crystalline solid and the disordered liquid. He labeled this state liquid crystal.
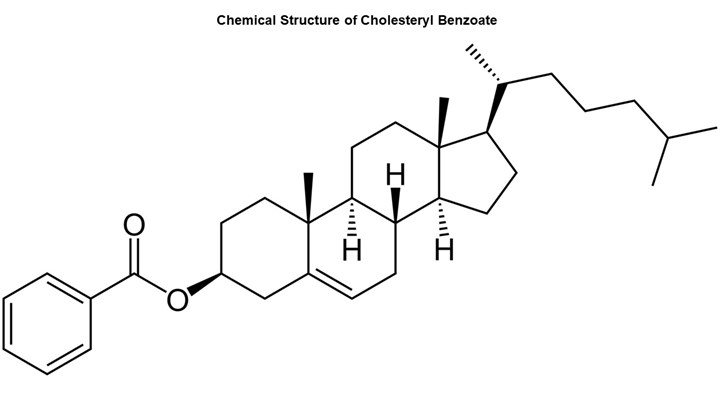
Cholesteryl benzoate is an ester that exhibits a high degree of aromatic character. This results in a rigid structure, and the chemical bonds are highly aligned. Modern LCPs share these structural characteristics. (Photo: Mike Sepe)
Fundamentally, solids generally organize into crystals with a shape and dimensions that are a function of the atoms or molecules that make up the substance. Analytical tools like X-ray diffraction are used to probe and characterize these structures, and thermal techniques can study the temperature and the quantity of energy required to break up these crystals and form the resulting liquid. It is understood that once the substance is in liquid form, the individual molecules can move freely relative to one another. Liquid crystals exhibit the fluidity normally associated with liquids, but at a molecular level there remains a significant degree of the long-range translational order typically found in solids.
To achieve this type of intermediate structure, a certain molecular geometry is required. The accompanying figure shows the chemical structure for cholesteryl benzoate, which is an ester. The molecule exhibits a high degree of aromatic character, which results in a rigid structure, and the chemical bonds are highly aligned. The modern liquid-crystal polymers share these structural characteristics. This allows the individual molecules to achieve a significant degree of orientation even when the temperature of the system is high enough to allow for independent molecular motion. This property of orientation is closely related to the ability of liquid crystal polymers to achieve very low viscosities and consequently fill very thin sections and at the same time to exhibit relatively low levels of strength at weld lines.
Most of the commercial LCP materials on the market today are based on polyester chemistry.
As is the case with all materials, LCPs derive their properties from their chemistry. The unique shape of molecules capable of forming liquid crystals can come from the main chain structure or from side-chain structure. In order to allow for melt processability, the structure must provide for a high degree of shear thinning to achieve manageable melt viscosities. Three forms of LCPs are recognized that reflect the structural aspects of how the liquid crystal forms in the melt state. These are known as nematic, smectic, and cholesteric. In nematic systems, the liquid crystals do not form a highly ordered system and the shape of the polymer molecule allows for a high degree of orientation. In the smectic materials there is a significant degree of layering within the structure that increases the viscosity of the material and inhibits orientation.
In the cholesteric structures, named after the discovery of this behavior in cholesterol-based materials, the chains twist into what can be envisioned as a spiral staircase shape. This prevents the orientation required for flow in the liquid-crystal state when molecular weight is great enough to achieve polymeric properties. Of these three, only the nematic structure achieves viscosities in the liquid-crystal state that allow for melt processing, most of which is done by injection molding.
As we have discussed, the first commercial LCP materials were aromatic polyamides (aramid) and known commercially as Kevlar, developed at DuPont in 1965. However, these materials have melting points that exceed their degradation temperature and consequently they cannot be melt processed.
The evolution of the current offerings of thermotropic LCP materials has been one of finding the balance between heat resistance and the ability to melt process the materials. Most of the commercial LCP materials on the market today are based on polyester chemistry. Homopolymers of p-hydroxybenzoic acid have melting points above 500°C (932°F), which places them outside the thermal capability of standard commercial molding machines. But incorporation of other monomers led to a variety of products with melting points that are within the achievable range of today’s processing equipment. We will review representative members of this commercial family in our next installment.
About the Author
Michael Sepe
Michael Sepe is an independent materials and processing consultant based in Sedona, Ariz., with clients throughout North America, Europe, and Asia. He has more than 45 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting and failure analysis. Contact: (928) 203-0408 • mike@thematerialanalyst.com
Related Content
How Do You Like Your Acetal: Homopolymer or Copolymer?
Acetal materials have been a commercial option for more than 50 years.
Read MoreWhat is the Allowable Moisture Content in Nylons? It Depends (Part 1)
A lot of the nylon that is processed is filled or reinforced, but the data sheets generally don’t account for this, making drying recommendations confusing. Here’s what you need to know.
Read MorePrices for All Volume Resins Head Down at End of 2023
Flat-to-downward trajectory for at least this month.
Read MoreTracing the History of Polymeric Materials: Acetal
The road from discovery in the lab to commercial viability can be long, and this was certainly the case for acetal polymers.
Read MoreRead Next
Tracing the History of Polymeric Materials, Part 26: High-Performance Thermoplastics
The majority of the polymers that today we rely on for outstanding performance — such as polysulfone, polyethersulfone, polyphenylsulfone and PPS — were introduced in the period between 1965 and 1985. Here’s how they entered your toolbox of engineering of materials.
Read MoreTracing the History of Polymeric Materials, Part 25: Silicones
The long road to the development of silicone resulted in a chemistry that is remarkably versatile.
Read MoreLead the Conversation, Change the Conversation
Coverage of single-use plastics can be both misleading and demoralizing. Here are 10 tips for changing the perception of the plastics industry at your company and in your community.
Read More.jpg;width=70;height=70;mode=crop)