3D Printing Biomaterials Make Push Into Medical Devices, Longer Term Implants
Group of companies is advancing biomaterials turning cellulose with thermoplastics made from sustainable resources into 3D printed medical devices.
It’s always gratifying to see an innovative, “young” company that you’ve reported on make further strides—either through expansion or technology licensing. In 2016, we reported on Innovative Plastics and Molding-IPM, Toledo, Ohio, and its focus on the development of 3D printing natural fibers (25% cellulose-based) in a polyolefin matrix to compete with PLA and ABS in the FDM additive manufacturing process.
IPM has since then licensed its Fibre Tuff technology for the medical market to startup FibreTuff Medical Biopolymers LLC, Toledo, Ohio. Moreover, a group of Northern Ohio companies, including FibreTuff Medical, is pioneering the advancement of biomaterials using cellulose with thermoplastics made from sustainable resources in 3D printing medical devices. A key one is PAPC—polyamide/PP/cellulose blends. This biomaterial is bio absorbable and not resorbable by the human body—differentiating it from standard biomaterials like PLA and PHB for the medical market. Led by the Center of Innovative Food Technology (CIFT), this nearly $500,000 collaboration is aiming to create manufacturing of Class I and Class II medical devices in Northwest Ohio.
Said FibreTuff Medical Bioppolymers’ CEO Tom Hughes, “Northwest Ohio is ideal for this innovation because of the close proximity to raw materials and the innovative approach to healthcare…this technology allows the creation of medical devices with incredible accuracy in shorter time, which enhances the overall recovery plan and way of life for a patient—not to mention lower medical costs for the hospital.”
FibreTuff will produce the advanced biomaterial PAPC for 3D printing Class I medical devices, such as foot orthotics, pediatric cases, helmets and filters. The FibreTuff PAPC has recently passed skin irritation and cytoxicity tests for these Class I devices performed by NAMAS—a medical regulatory testing company. It has also been shown to have no offensive odors, lower processing costs, unique surface texture, improved colorability and chemical resistance.
Initially, FibreTuff will commercialize 3D filaments in the grant collaboration for 3D printing of Class I wearables for Merch Health in Toledo. Added Hughes, “The plan would be to eventually use PAPC for Class II devices—items that are allowed to remain inside the body for 20 days, such as implants for pacemakers made out of sustainable cellulose. Some day, sustainable biomedical devices could remain in a person’s body forever…although that will come at a later time. 3D printing opens up a chole new arena of creative and inventive possibilities,” said Hughes.
The statewide project is in part funded by the Ohio Manufacturing Extension Partnership (Ohio MEP) through an Advanced Manufacturing Partnership (AMP) grant from the State of Ohio. It will be executed in three phases by:
- Qualifying three different biomaterial formulas for the 3D printing process.
- Designing and 3D printing medical devices using three biomaterials.
- Developing conceptual prototypes for “smart” devices using 3D printed designs.
The partnership involves the following key players at various stages of the project:
- FibreTuff will use natural ingredients that promote sustainable materials.
- Whiteside Orthotics and Prosthetics Group of Youngstown, will design and test medical devices and collaborate in the design of “smart devices”.
- JuggerBot3D, Youngstown, and Valtronic, Solon, will produce devices.
- Valtronic will provide expertise and production capabilities due to their background in complex electronic devices used in the medical industry.
- Dr. Eric MacDonald of Youngstown State University will collaborate in the design of “smart devices”.
- Mercy Health, Toledo, will serve as the regional healthcare partner to prototype 3D printed medical devices for the benefit of future patients.
Ohio MEP’s aims to drive productivity, innovation and global competitiveness for Ohio manufacturers with a focus on small and medium sized enterprises.
Related Content
3D Printing of Injection Molds Flows in a New Direction
Hybrids of additive manufacturing and CNC machining can shorten tooling turnaround times.
Read MoreProduction Tool, Prototype Time
Mantle's metal 3D printing technology targeted toolmaking and injection molders and moldmakers are taking notice.
Read MoreCustom Molder Manages Growth on Several Fronts
Adding people, plants and machines, expanding capabilities in LSR, high-tonnage presses, automation and 3D printing—EVCO Plastics maintains momentum through challenging times.
Read MoreKraussMaffei Launches Two Additive Manufacturing Lines at K 2022
Long established in injection molding, extrusion and polyurethane reaction process machinery, 184-yr-old KraussMaffei prepares to enter the industrial additive manufacturing market.
Read MoreRead Next
Troubleshooting Screw and Barrel Wear in Extrusion
Extruder screws and barrels will wear over time. If you are seeing a reduction in specific rate and higher discharge temperatures, wear is the likely culprit.
Read MoreWhy (and What) You Need to Dry
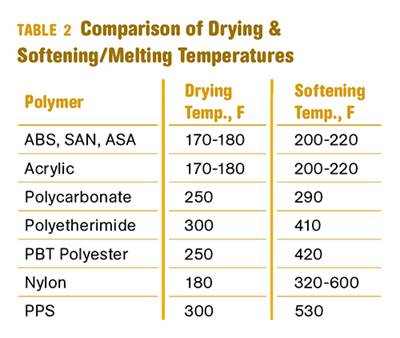
Other than polyolefins, almost every other polymer exhibits some level of polarity and therefore can absorb a certain amount of moisture from the atmosphere. Here’s a look at some of these materials, and what needs to be done to dry them.
Read MoreHow Polymer Melts in Single-Screw Extruders
Understanding how polymer melts in a single-screw extruder could help you optimize your screw design to eliminate defect-causing solid polymer fragments.
Read More