Nylon 66 Resins for High-Performance Medical Devices
Ascend and Foster announce joint marketing of new Hi-Dura Med grade portfolio.
Photo Credit: Foster Corp. Distribution and Ascend Performance Materials
A portfolio of nylon 66-based compounds for high-performance medical devices was recently launched jointly by Ascend Performance Materials and Foster Corp.’s Distribution business. According to Foster Distribution’s president Larry Johnson, Foster worked closely with Ascend in the development of the new Hi-Dura Med product line and is pleased to be one of Ascend’s marketing partners for these products. The initial Ascend medical product line launch consists of three products, all of which have been tested for compliance to ISO 10993-5.
▪ Hi-Dura Med AG33 NT0862 (glass-filled nylon 66)
▪ Hi-Dura Med AI1 NT0861 (impact-modified nylon 66)
▪ Hi-Dura Med AP NT0860 (lubricated-unfilled nylon 66)
Ascend plans to launch further medical-grade products in 2023 to complement and expand the initial product line. Said Ascend’s global healthcare business manager Dhruv Shah, “Foster Corporation is a well- known name in the medical device industry with a strong reputation of providing quality products and technical service. Ascend is proud to have established this relationship and we look forward to growing our healthcare business with their support.”
Related Content
-
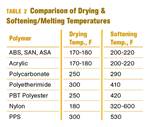
What is the Allowable Moisture Content in Nylons? It Depends (Part 1)
A lot of the nylon that is processed is filled or reinforced, but the data sheets generally don’t account for this, making drying recommendations confusing. Here’s what you need to know.
-
Prices for All Volume Resins Head Down at End of 2023
Flat-to-downward trajectory for at least this month.
-
PBT and PET Polyester: The Difference Crystallinity Makes
To properly understand the differences in performance between PET and PBT we need to compare apples to apples—the semi-crystalline forms of each polymer.











.png;maxWidth=300;quality=90)