When you’ve experienced a steady run of double-digit growth only to get sidetracked by a global pandemic, you might expect it to take awhile for business to bounce back. But for Foster Corp., which focuses on the medical market, business has come back at a furious pace. Fortunately, this compounder and distributor, headquartered in Putnam, Conn., was prepared.
On one hand, it might be surprising to learn that a company whose business is primarily medical would have a zero-growth year during the COVID-19 crisis, when demand for medical products was robust. But a large portion of Foster’s customized compounds are sold to extruders of medical tubing. That particular segment of the medical market took a hit last year when hospitals worldwide put a hold on elective surgeries. Now that those restrictions have started to be lifted, Foster’s business is going gangbusters. “In the first half of this year we had a total of 5000 orders,” notes Lawrence Acquarulo, CEO of Foster, which has plants in both Connecticut and Las Vegas. “That’s tracking 20% above last year.”
He adds, “We’ve been growing every year since we started, usually by double digits. There were two notable exceptions: 2002, because of 9/11; and last year, where we experienced zero growth because of COVID. But we have an exceptional workforce, and we had customer programs already established, so that we were ready when things picked up. And things picked up very quickly.”
Acquarulo estimates his business to be 90% medical. “We supply the majority of medical-tubing processors,” he states. “In many cases we are their primary supplier. We also supply medical molders that make parts such as connectors, bone screws, surgical implants, and packaging. We have more than 900 active customers. These range from big medical OEMs to medical extrusion and molding operators to a doctor who might call us with an idea for a new product.”

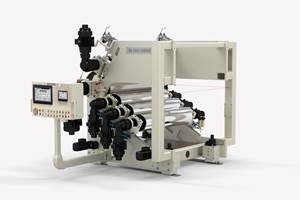
A new plant and new production capacity have helped Foster keep pace with orders that to date have outpaced last year’s by 20%, says CEO Larry Acquarulo.
Foster grows by not only securing new customers, but by getting new projects from its existing client base. Since March 2021, Foster has been operating on a 24:7 basis. It now employs 150 in Connecticut and has job openings for 12 more people to fill.
Acquarulo started Foster in 1989 in a 2500 ft² building with one machine in Putnam. Today, Foster is one of North America’s largest suppliers of compounds for minimally invasive medical devices, mainly catheters for interventional radiology and cardiology. Before starting Foster, Acquarulo worked for Imperial Chemical Industries and LNP. “We learned a lot about physical properties and flow characteristics of materials back then. The medical-device market was starting to emerge, and we saw an opportunity to start a business where quality and engineering support were highly valued.”
When Plastics Technology last visited Foster in 2011, its footprint in Connecticut consisted of a 46,000-ft² facility with 85 employees. That building now houses seven twin-screw extruders, mostly from Leistritz, ranging in size from 16 mm to 53 mm. It also is equipped with single-screw extruders from Davis-Standard, which are used for product development, making rods, films, or sheet.
“We supply 80-90% of medical-tubing processors.”
In 2015, Foster added a 32,000 ft² building on the site to produce drug-delivery systems via twin-screw extrusion. Foster Delivery Science (FDS) combines water-soluble polymers with active pharmaceutical ingredients (API) for drug companies, primarily for pre-clinical and clinical trials. FDS has two Leistritz twin-screws, three American Kuhne single-screw extruders, two cryogenic Fitz Mills, three cleanrooms, an R&D bay, a certified API sampling room, an analytical lab, and a press to produce tablets.

Foster’s new plant will house two twin-screw compounding lines dedicated to longer runs.
“FDS’ niche is mainly supporting pharmaceutical companies in the early stages of development of medications,” Acquarulo explains. “Sometimes we provide just 50 g of material. In other cases, we provide clinical production quantities of 100 g up to 100 kg.”
Just prior to the pandemic, Foster opened a third building on its campus. On Plastics Technology’s recent visit, a 50-mm twin was being moved from the main plant to this new 56,000 ft2 facility, which was also preparing for delivery of a 40-mm Leistritz MAXX twin. “The new plant is set up specifically to accommodate longer-run orders,” Acquarulo explains. “Our original building continues to focus on a wide mix of shorter, higher-complexity runs, and our operation is designed to facilitate quick turnarounds.”
All told, the third building repesents a $9.5 million investment for Foster. “We were busting at the seams,” Acquarulo states. “To support our distribution business, we were renting a warehouse in Auburn, Mass. I didn’t want to pay rent anymore.” The new facility also features a large training room and a toolroom, where it maintains its pelletizing dies and fabricates its own jigs and fixtures.
“Our original building will continue to handle a wide mix of shorter, higher-complexity runs.”
Foster’s Putnam campus also features a class 10,000 cGMP clean room equipped with GMP compliant extrusion equipment, highly accurate feeders, and fully integrated computer process controls and monitoring systems for manufacturing implantable materials. Foster also recently added a 100-ton Sodick Plustech injection molding machine to make stock-shape samples and prototypes. It also has two 30-ton Arburg presses or preparing color chips and test specimens.

Foster’s has a dedicated room in its new plant for material drying.
“Our recent growth necessitated the new building as both our compounding and distribution businesses have reached the point that space would become a limiting factor if we didn’t make this move,” Acquarulo states. “The new building will not only allow our growth to continue, but it will also allow us to grow in new markets where we haven’t been able to compete in the past, including larger-volume medical applications.”
In each building, Foster’s production operations are supported by sophisticated material blending and feeding technologies, which include high-intensity premixers, loss-in-weight pellet and powder feeders, and liquid feeders. It uses dry blending for applications that have less rigorous performance or appearance requirements and a greater emphasis on cost. Depending on the size of the order, the compound can be blended inline or batched offline in a separate room.
Batches made there are fully traceable. Ingredients are barcoded to ensure batching accuracy and allow for data collection. Foster has full color-matching capabilities covering thousands of PMS color specifications and countless custom shades. Biocompatible organic and inorganic pigments can be added to unmodified polymers or custom compounds.
Production lines are sometimes equipped with inline weighing and packaging right from the pelletizer. Foster custom built its production-monitoring system for tracking parameters such as extruder temperatures and pressures; blender feed rates; and screw rpm and torque on each line. This allows the recipe and process parameters to be duplicated for the next run, as well as providing both Foster and its customers with the tracking they may require.
Foster has banks of central and machine-mounted dryers from Novatec and Dri-Air, as well as a Universal Dynamics crystallizer for PET. Post-extrusion technologies such as strand and underwater pelletizers, sifters, classifiers, and cross-tumblers ensure finished materials are consistent throughout the lot and from lot to lot.
“We solve problems. We offer on-time delivery. We are responsive to the special needs of the medical-device community.”
The company has expertise in a broad range of polymers. It is considered a leader in polymer compounds for minimally invasive devices, including diagnostic and therapeutic catheters, many of which are TPE-based. It compounds a gamut of fluoropolymers on twin-screw extruders furnished with corrosion-resistant Inconel barrels and Hastelloy metal coatings on all contact surfaces.
Foster also routinely processes PEBA elastomers, TPUs, nylon 11 and 12, ABS, PC and a slew of high-performance materials that include PEEK, PEI and PSU. It has expertise in compounding bioresorbable polymers that include PLA, polyglycolide (PGA) and copolymers of PLA/PGA that it tailors to meet required mechanical performance and resorption rates. These materials can be formulated with bone-growth additives such as tricalcium phosphate and hydroxyapatite.

With the additional business, Foster can now focus production of shorter, highly complex runs at its original site in Connecticut.
For catheters in particular, Foster has developed a line of LoPro radiopaque compounds with fillers based on bismuth subcarbonate, barium sulfate, bismuth oxychloride, bismuth trioxide, tungsten and others. ts ProPell line of low-friction compounds are for molded and extruded medical devices and components that require low coefficient of friction and are formulated with perfluoropolyether synthetic oil or PTFE powder. Foster has also developed a range of antimicrobial technologies using both silver and polymer-based agents to fight infection and prevent development of bacteria on contact surfaces.
The firm’s base in Connecticut is supported by a 30,000 ft2 plant in Las Vegas with similar capabilities. It’s equipped with four twins ranging from 30 mm to 40 mm, with a new 40 mm on order from Leistritz. Customers on the West Coast, Central America and Asia are served from here.
The ‘Original Game Plan’
Foster’s Polymer Distribution business specializes in providing high-performance polymers and additives to the medical market, including class I, II and III devices. Its portfolio contains a wide range of specialty resins, manufactured by material suppliers including Arkema, Eastman, Mitsubishi, Repsol, Resirene and Shepherd Chemical. It also has a distribution agreement with Drake Medical Plastics of Texas, which provides “resin-to-shape” polymer conversion into rod, plate, and tube for machined components used in the medical and life-science industries.

Foster mirrors its Connecticut production capabilities, including color formulating and compounding, at its Las Vegas plant.
In all of these relationships, Foster identifies specific material grades to meet what the application needs. “Our goal is to discover, develop and assist in bringing new applications to fruition,” says Acquarulo. “Using our industry knowledge and experience, we can leverage the technology platforms of our partners to assist in the qualification of the next generation of medical devices.”
New buildings, capabilities, and distribution arrangements notwithstanding, Acquarulo insists that the company he started in 1989 “still works off the original game plan. We solve problems. We offer on-time delivery. We are responsive to the special needs of the medical-device community.”
Related Content
How to Maintain Pelletizing Quality When Acid Attacks
Developments in the chemistry of polymers and additives have made corrosion a real problem in pelletizers. Here’s how to ward it off.
Read MoreGreen’s the Theme in Extrusion/Compounding
The drive toward circular economy is requiring processors to make more use of PCR. Machine builders at K—across all extrusion processes—will be highlighting innovations to help them do just that.
Read MoreWhat to Know About Your Materials When Choosing a Feeder
Feeder performance is crucial to operating extrusion and compounding lines. And consistent, reliable feeding depends in large part on selecting a feeder compatible with the materials and additives you intend to process. Follow these tips to analyze your feeder requirements.
Read MoreHow to Configure Your Twin-Screw Extruder: Part 3
The melting mechanism in a twin-screw extruder is quite different from that of a single screw. Design of the melting section affects how the material is melted, as well as melt temperature and quality.
Read MoreRead Next
On Site: Tailoring Medical Solutions… Sometimes in 50-lb Lots
Smaller volumes teams with higher quality and value as keys to the success of this growing medical compounder.
Read MoreFoster Now Distributor of Resirene's SMMA Copolymers
As exclusive distributor of Resirene’s SMMA, Foster will also now offer custom compounds based on SMMA including custom colors and impact-modified versions.
Read MoreFoster Breaks Ground on New Plant Expansion for Medical-Grade Polymers
Strong growth in the medical and pharmaceutical market sectors drives Foster to expand.
Read More













.png;maxWidth=300;quality=90)