Acetal Resin Series for Medical Applications
Polyplastics’ Duracon POM PM series launched globally.
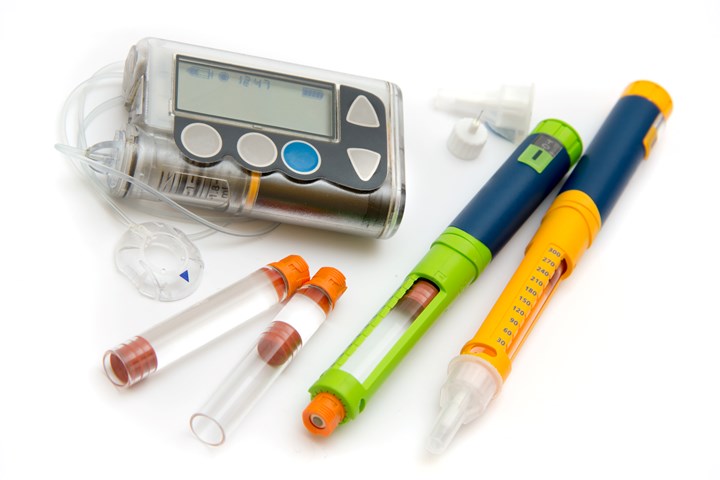
Commercial availability of a new series of acetal resins for medical applications from the Polyplastics Group has been launched. The company will supply Duracon POM PM (Polyoxymethylene/Acetal) series to all global regions including Asia such as China and India, and Europe and the U.S. Polyplastics has served the medical and healthcare field for decades with its high-purity Topas COC (cyclic olefin copolymer). As the world’s leading POM manufacturer, Polyplastics now adds the PM Series to its medical grade portfolio and plans to expand material supply to the medical and healthcare market.
Polyplastics’ two acetal grades include a standard viscosity grade, PM09S01N, which delivers the reliable mechanical properties and moldability expected from POM. In addition, a high-flow grade, PM27S01N, will enable wall-thinning, miniaturization, and lightweighting of medical devices that are becoming more complex and highly functional. Both grades meet medical device manufacturers’ key requirements and are targeted for a wide range of applications such as drug contact and delivery. Both also offer global market regulatory compliance including ISO 10993 and USP Class VI biocompatibility/cytotoxicity, FDA Drug Master File (DMF) and Device Master File (MAF), and EU 10/2011 and FDA food contact 21 CFR 177.2470. The materials can be sterilized under hot steam and ethylene oxide (EtO) sterilization conditions.
Related Content
-
What's the Allowable Moisture Content in Nylons? It Depends: Part 2
Operating within guidelines from material suppliers can produce levels of polymer degradation. Get around it with better control over either the temperature of the melt or the barrel residence time.
-
Melt Flow Rate Testing–Part 1
Though often criticized, MFR is a very good gauge of the relative average molecular weight of the polymer. Since molecular weight (MW) is the driving force behind performance in polymers, it turns out to be a very useful number.
-
Tracing the History of Polymeric Materials: Acetal
The road from discovery in the lab to commercial viability can be long, and this was certainly the case for acetal polymers.